Contribution of cysteine residues in the extracellular domain of the F protein of human respiratory syncytial virus to its function
- PMID: 16723026
- PMCID: PMC1540417
- DOI: 10.1186/1743-422X-3-34
Contribution of cysteine residues in the extracellular domain of the F protein of human respiratory syncytial virus to its function
Abstract
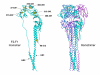
The mature F protein of all known isolates of human respiratory syncytial virus (HRSV) contains fifteen absolutely conserved cysteine (C) residues that are highly conserved among the F proteins of other pneumoviruses as well as the paramyxoviruses. To explore the contribution of the cysteines in the extracellular domain to the fusion activity of HRSV F protein, each cysteine was changed to serine. Mutation of cysteines 37, 313, 322, 333, 343, 358, 367, 393, 416, and 439 abolished or greatly reduced cell surface expression suggesting these residues are critical for proper protein folding and transport to the cell surface. As expected, the fusion activity of these mutations was greatly reduced or abolished. Mutation of cysteine residues 212, 382, and 422 had little to no effect upon cell surface expression or fusion activity at 32 degrees C, 37 degrees C, or 39.5 degrees C. Mutation of C37 and C69 in the F2 subunit either abolished or reduced cell surface expression by 75% respectively. None of the mutations displayed a temperature sensitive phenotype.
Figures



References
-
- Ison MG, Hayden FG. Viral infections in immunocompromised patients: what's new with respiratory viruses? Curr Opin Infect Dis. 2002;15:355–367. - PubMed
-
- Whimbey E, Ghosh S. Respiratory syncytial virus infections in immunocompromised adults. Curr Clin Top Infect Dis. 2000;20:232–255. - PubMed
-
- Collins PL, Chanock RM, Murphy BR. Respiratory syncytial virus. In: Knipe DM and Howley PM, editor. Fields Virology. Fourth. Vol. 1. Philadelphia, Lippincott, Williams, and Wilkins; 2001. pp. 1443–1485.
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

