Stress increases vulnerability to inflammation in the rat prefrontal cortex
- PMID: 16723527
- PMCID: PMC6675274
- DOI: 10.1523/JNEUROSCI.0802-06.2006
Stress increases vulnerability to inflammation in the rat prefrontal cortex
Abstract
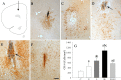
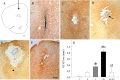
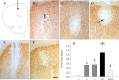
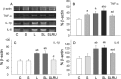
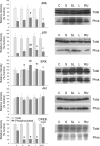
Inflammation could be involved in some neurodegenerative disorders that accompany signs of inflammation. However, because sensitivity to inflammation is not equal in all brain structures, a direct relationship is not clear. Our aim was to test whether some physiological circumstances, such as stress, could enhance susceptibility to inflammation in the prefrontal cortex (PFC), which shows a relative resistance to inflammation. PFC is important in many brain functions and is a target for some neurodegenerative diseases. We induced an inflammatory process by a single intracortical injection of 2 microg of lipopolysaccharide (LPS), a potent proinflammogen, in nonstressed and stressed rats. We evaluated the effect of our treatment on inflammatory markers, neuronal populations, BDNF expression, and behavior of several mitogen-activated protein (MAP) kinases and the transcription factor cAMP response element-binding protein. Stress strengthens the changes induced by LPS injection: microglial activation and proliferation with an increase in the levels of the proinflammatory cytokine tumor necrosis factor-alpha; loss of cells such as astroglia, seen as loss of glial fibrillary acidic protein immunoreactivity, and neurons, studied by neuronal-specific nuclear protein immunohistochemistry and GAD67 and NMDA receptor 1A mRNAs expression by in situ hybridization. A significant increase in the BDNF mRNA expression and modifications in the levels of MAP kinase phosphorylation were also found. In addition, we observed a protective effect from RU486 [mifepristone (11beta-[p-(dimethylamino)phenyl]-17beta-hydroxy-17-(1-propynyl)estra-4,9-dien-3-one)], a potent inhibitor of the glucocorticoid receptor activation. All of these data show a synergistic effect between inflammation and stress, which could explain the relationship described between stress and some neurodegenerative pathologies.
Figures

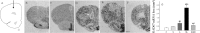


References
-
- Abekawa T, Ohmori T, Ito K, Koyama T (2000). D1 dopamine receptor activation reduces extracellular glutamate and GABA concentrations in the medial prefrontal cortex. Brain Res 867:250–254. - PubMed
-
- Arnsten AF (1998). The biology of being frazzled. Science 280:1711–1712. - PubMed
-
- Behl C, Trapp T, Skutella T, Holsboer F (1997a). Protection against oxidative stress-induced neuronal cell death—a novel role for RU486. Eur J Neurosci 9:912–920. - PubMed
-
- Behl C, Lezoualc’h F, Trapp T, Widmann M, Skutella T, Holsboer F (1997b). Glucocorticoids enhance oxidative stress-induced cell death in hippocampal neurons in vitro. Endocrinology 138:101–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
