Silencing acid-sensing ion channel 1a alters cone-mediated retinal function
- PMID: 16723538
- PMCID: PMC6675265
- DOI: 10.1523/JNEUROSCI.0344-06.2006
Silencing acid-sensing ion channel 1a alters cone-mediated retinal function
Abstract
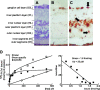
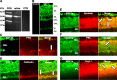
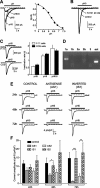
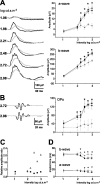
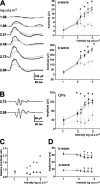
The action of extracellular protons on retinal activity and phototransduction occurs through pH-sensitive elements, mainly membrane conductances present on the different cell types of the outer and inner nuclear layers and of the ganglion cell layer. Acid-sensing ion channels (ASICs) are depolarizing conductances that are directly activated by protons. We investigated the participation of ASIC1a, a particular isoform of ASICs, in retinal physiology in vivo using electroretinogram measurements. In situ hybridization and immunohistochemistry localized ASIC1a in the outer and inner nuclear layers (cone photoreceptors, horizontal cells, some amacrine and bipolar cells) and in the ganglion cell layer. Both the in vivo knockdown of ASIC1a by antisense oligonucleotides and the in vivo blocking of its activity by PcTx1, a specific venom peptide, were able to decrease significantly and reversibly the photopic a- and b-waves and oscillatory potentials. Our study indicates that ASIC1a is an important channel in normal retinal activity. Being present in the inner segments of cones and inner nuclear layer cells, and mainly at synaptic cleft levels, it could participate in gain adaptation to ambient light of the cone pathway, facilitating cone hyperpolarization in brightness and modulating synaptic transmission of the light-induced visual signal.
Figures


References
-
- Akopian AN, Chen CC, Ding Y, Cesare P, Wood JN (2000). A new member of the acid-sensing ion channel family. NeuroReport 11:2217–2222. - PubMed
-
- Ayoub GS, Korenbrot JI, Copenhagen DR (1989). Release of endogenous glutamate from isolated cone photoreceptors of the lizard. Neurosci Res Suppl 10:S47–S55. - PubMed
-
- Barnett NL, Pow DV (2000). Antisense knockdown of GLAST, a glial glutamate transporter, compromises retinal function. Invest Ophthalmol Vis Sci 41:585–591. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
