Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa
- PMID: 16723551
- PMCID: PMC1479097
- DOI: 10.1128/AAC.00035-06
Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa
Abstract
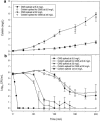
There is a dearth of information on the pharmacodynamics of "colistin," despite its increasing use as a last line of defense for treatment of infections caused by multidrug-resistant gram-negative organisms. The antimicrobial activities of colistin and colistin methanesulfonate (CMS) were investigated by studying the time-kill kinetics of each against a type culture of Pseudomonas aeruginosa in cation-adjusted Mueller-Hinton broth. The appearance of colistin from CMS spiked at 8.0 and 32 mg/liter was measured by high-performance liquid chromatography, which generated colistin concentration-time profiles. These concentration-time profiles were subsequently mimicked in other incubations, independent of CMS, by incrementally spiking colistin. When the cultures were spiked with CMS at either concentration, there was a substantial delay in the onset of the killing effect which was not evident until the concentrations of colistin generated from the hydrolysis of CMS had reached approximately 0.5 to 1 mg/liter (i.e., approximately 0.5 to 1 times the MIC for colistin). The time course of the killing effect was similar when colistin was added incrementally to achieve the same colistin concentration-time course observed from the hydrolysis of CMS. Given that the killing kinetics of CMS can be accounted for by the appearance of colistin, CMS is an inactive prodrug of colistin with activity against P. aeruginosa. This is the first study to demonstrate the formation of colistin in microbiological media containing CMS and to demonstrate that CMS is an inactive prodrug of colistin. These findings have important implications for susceptibility testing involving "colistin," in particular, for MIC measurement and for microbiological assays and pharmacokinetic and pharmacodynamic studies.
Figures
References
-
- Bergan, T., and J. Fuglesang. 1982. Polymyxin antibiotics: chemical and pharmacokinetic properties. Antibiot. Chemother. 31:119-144. - PubMed
-
- Berlana, D., J. M. Llop, E. Fort, M. B. Badia, and R. Jodar. 2005. Use of colistin in the treatment of multiple-drug-resistant gram-negative infections. Am. J. Health-Syst. Pharm. 62:39-47. - PubMed
-
- Boutiba-Ben Boubaker, I., J. Boukadida, O. Triki, N. Hannachi, and S. Ben Redjeb. 2003. Outbreak of nosocomial urinary tract infections due to a multidrug resistant Pseudomonas aeruginosa. Pathol. Biol. 51:147-150. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

