Loss of angiotensin-converting enzyme-2 leads to the late development of angiotensin II-dependent glomerulosclerosis
- PMID: 16723697
- PMCID: PMC1606622
- DOI: 10.2353/ajpath.2006.051091
Loss of angiotensin-converting enzyme-2 leads to the late development of angiotensin II-dependent glomerulosclerosis
Abstract
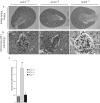
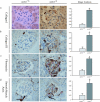
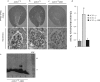
Angiotensin-converting enzyme-2 (ACE2), a membrane-bound carboxymonopeptidase highly expressed in the kidney, functions as a negative regulator of the renin-angiotensin system. Here we report early accumulation of fibrillar collagen in the glomerular mesangium of male ACE2 mutant (ACE2-/y) mice followed by development of glomerulosclerosis by 12 months of age whereas female ACE2 mutant (ACE2-/-) mice were relatively protected. Progressive kidney injury was associated with increased deposition of collagen I, collagen III and fibronectin in the glomeruli and increased urinary albumin excretion compared to age-matched control mice. These structural and functional changes in the glomeruli of male ACE2 mutant mice were prevented by treatment with the angiotensin II type-1 receptor antagonist irbesartan. Loss of ACE2 was associated with a marked increase in renal lipid peroxidation product formation and activation of mitogen-activated protein kinase and extracellular signal-regulated kinases 1 and 2 in glomeruli, events that are also prevented by angiotensin II type-1 receptor blockade. We conclude that deletion of the ACE2 gene leads to the development of angiotensin II-dependent glomerular injury in male mice. These findings have important implications for our understanding of ACE2, the renin-angiotensin system, and gender in renal injury, with ACE2 likely to be an important therapeutic target in kidney disease.
Figures



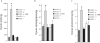


References
-
- Taal MW, Brenner BM. Renoprotective benefits of RAS inhibition: from ACEI to angiotensin II antagonists. Kidney Int. 2000;57:1803–1817. - PubMed
-
- Zaman MA, Oparil S, Calhoun DA. Drugs targeting the renin-angiotensin-aldosterone system. Nat Rev Drug Discov. 2002;1:621–636. - PubMed
-
- Oudit GY, Crackower MA, Backx PH, Penninger JM. The role of ACE2 in cardiovascular physiology. Trends Cardiovasc Med. 2003;13:93–101. - PubMed
-
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD, The Collaborative Study Group The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–1462. - PubMed
-
- Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, Ponticelli C, Ritz E, Zucchelli P, The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. N Engl J Med. 1996;334:939–945. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

