Isolation of a novel population of multipotent adult stem cells from human hair follicles
- PMID: 16723703
- PMCID: PMC1606635
- DOI: 10.2353/ajpath.2006.051170
Isolation of a novel population of multipotent adult stem cells from human hair follicles
Abstract
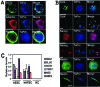
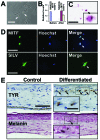
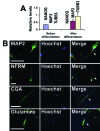
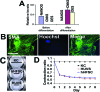
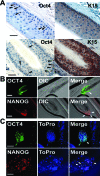
Hair follicles are known to contain a well-characterized niche for adult stem cells: the bulge, which contains epithelial and melanocytic stem cells. Using human embryonic stem cell culture conditions, we isolated a population of adult stem cells from human hair follicles that are distinctively different from known epithelial or melanocytic stem cells. These cells do not express squamous or melanocytic markers but express neural crest and neuron stem cell markers as well as the embryonic stem cell transcription factors Nanog and Oct4. These precursor cells proliferate as spheres, are capable of self-renewal, and can differentiate into multiple lineages. Differentiated cells not only acquire lineage-specific markers but also demonstrate appropriate functions in ex vivo conditions. Most of the Oct4-positive cells in human skin were located in the area highlighted by cytokeratin 15 staining in vivo. Our data suggest that human embryonic stem cell medium can be used to isolate and expand human adult stem cells. Using this method, we isolated a novel population of multipotent adult stem cells from human hair follicles, and these cells appear to be located in the bulge area. Human hair follicles may provide an accessible, autologous source of adult stem cells for therapeutic application.
Figures

References
-
- Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–537. - PubMed
-
- Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlstrom H, Lendahl U, Frisen J. Generalized potential of adult neural stem cells. Science. 2000;288:1660–1663. - PubMed
-
- Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. - PubMed
-
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. - PubMed
-
- Orlic D. Adult bone marrow stem cells regenerate myocardium in ischemic heart disease. Ann NY Acad Sci. 2003;996:152–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

