Multiorgan autoimmune inflammation, enhanced lymphoproliferation, and impaired homeostasis of reactive oxygen species in mice lacking the antioxidant-activated transcription factor Nrf2
- PMID: 16723711
- PMCID: PMC1606627
- DOI: 10.2353/ajpath.2006.051113
Multiorgan autoimmune inflammation, enhanced lymphoproliferation, and impaired homeostasis of reactive oxygen species in mice lacking the antioxidant-activated transcription factor Nrf2
Abstract
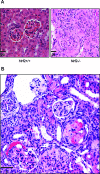
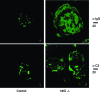
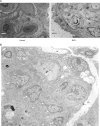
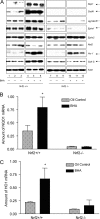
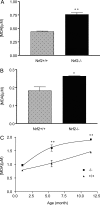
Nuclear factor erythroid 2-related factor 2 (Nrf2) is an antioxidant-activated cap "n" collar basic leucine zipper transcription factor. To assess the function of Nrf2 in the antioxidant response, we examined mice with targeted disruption of the Nrf2 gene. Nrf2-null mice developed complex disease manifestations, with a majority exhibiting a lupus-like autoimmune syndrome characterized by multiorgan inflammatory lesions with a marked female predominance, appearance of anti-double-stranded DNA antibodies in young adulthood, intravascular deposition of immunoglobulin complexes in blood vessels, and premature death due to rapidly progressing membranoproliferative glomerular nephritis. Mechanistic analyses revealed that the null mice showed enhanced proliferative response of CD4+ T cells, altered ratios of CD4+ and CD8+ cells, and increased oxidative lesions in tissues. Analyses of antioxidant-induced gene expression showed that the knockout mice were devoid of the basal and inducible expression of certain phase 2 detoxification enzymes and antioxidant genes in hepatic and lymphoid cells in vivo. Our findings suggest that Nrf2 mediates important antioxidant functions involved in the control of peripheral lymphocyte homeostasis and autoimmune surveillance.
Figures
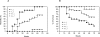






References
-
- Mohler J, Vani K, Leung S, Epstein A. Segmentally restricted, cephalic expression of a leucine zipper gene during Drosophila embryogenesis. Mech Dev. 1991;34:3–9. - PubMed
-
- Chan JY, Cheung MC, Moi P, Chan K, Kan YW. Chromosomal localization of the human NF-E2 family of bZIP transcription factors by fluorescence in situ hybridization. Hum Genet. 1995;95:265–269. - PubMed
-
- Andrews NC, Erdjument-Bromage H, Davidson MB, Tempst P, Orkin SH. Erythroid transcription factor NF-E2 is a haematopoietic-specific basic-leucine zipper protein. Nature. 1993;362:722–728. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

