Retrovirus infection strongly enhances scrapie infectivity release in cell culture
- PMID: 16724107
- PMCID: PMC1500854
- DOI: 10.1038/sj.emboj.7601162
Retrovirus infection strongly enhances scrapie infectivity release in cell culture
Abstract
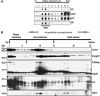
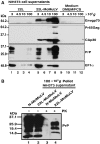
Prion diseases are neurodegenerative disorders associated in most cases with the accumulation in the central nervous system of PrPSc (conformationally altered isoform of cellular prion protein (PrPC); Sc for scrapie), a partially protease-resistant isoform of the PrPC. PrPSc is thought to be the causative agent of transmissible spongiform encephalopathies. The mechanisms involved in the intercellular transfer of PrPSc are still enigmatic. Recently, small cellular vesicles of endosomal origin called exosomes have been proposed to contribute to the spread of prions in cell culture models. Retroviruses such as murine leukemia virus (MuLV) or human immunodeficiency virus type 1 (HIV-1) have been shown to assemble and bud into detergent-resistant microdomains and into intracellular compartments such as late endosomes/multivesicular bodies. Here we report that moloney murine leukemia virus (MoMuLV) infection strongly enhances the release of scrapie infectivity in the supernatant of coinfected cells. Under these conditions, we found that PrPC, PrPSc and scrapie infectivity are recruited by both MuLV virions and exosomes. We propose that retroviruses can be important cofactors involved in the spread of the pathological prion agent.
Figures






Similar articles
-
Mouse neuroblastoma cells release prion infectivity associated with exosomal vesicles.Biol Cell. 2008 Oct;100(10):603-15. doi: 10.1042/BC20080025. Biol Cell. 2008. PMID: 18422484
-
Co-infection with the friend retrovirus and mouse scrapie does not alter prion disease pathogenesis in susceptible mice.PLoS One. 2012;7(1):e30872. doi: 10.1371/journal.pone.0030872. Epub 2012 Jan 25. PLoS One. 2012. PMID: 22295118 Free PMC article.
-
KDEL-tagged anti-prion intrabodies impair PrP lysosomal degradation and inhibit scrapie infectivity.Biochem Biophys Res Commun. 2005 Dec 30;338(4):1791-7. doi: 10.1016/j.bbrc.2005.10.146. Epub 2005 Nov 2. Biochem Biophys Res Commun. 2005. PMID: 16288721
-
Prion encephalopathies of animals and humans.Dev Biol Stand. 1993;80:31-44. Dev Biol Stand. 1993. PMID: 8270114 Review.
-
Prion diseases of the central nervous system.Monogr Pathol. 1990;(32):86-122. Monogr Pathol. 1990. PMID: 2192281 Review.
Cited by
-
Efficient inhibition of infectious prions multiplication and release by targeting the exosomal pathway.Cell Mol Life Sci. 2015 Nov;72(22):4409-27. doi: 10.1007/s00018-015-1945-8. Epub 2015 Jun 6. Cell Mol Life Sci. 2015. PMID: 26047659 Free PMC article.
-
Highly efficient intercellular spreading of protein misfolding mediated by viral ligand-receptor interactions.Nat Commun. 2021 Oct 19;12(1):5739. doi: 10.1038/s41467-021-25855-2. Nat Commun. 2021. PMID: 34667166 Free PMC article.
-
Identification of an inhibitory budding signal that blocks the release of HIV particles and exosome/microvesicle proteins.Mol Biol Cell. 2011 Mar 15;22(6):817-30. doi: 10.1091/mbc.E10-07-0625. Epub 2011 Jan 19. Mol Biol Cell. 2011. PMID: 21248205 Free PMC article.
-
The first non-prion pathogen identified: neurotropic influenza virus.Prion. 2022 Dec;16(1):1-6. doi: 10.1080/19336896.2021.2015224. Prion. 2022. PMID: 34978525 Free PMC article.
-
Bovine spongiform encephalopathy infection alters endogenous retrovirus expression in distinct brain regions of cynomolgus macaques (Macaca fascicularis).Mol Neurodegener. 2011 Jun 23;6(1):44. doi: 10.1186/1750-1326-6-44. Mol Neurodegener. 2011. PMID: 21699683 Free PMC article.
References
-
- Basyuk E, Galli T, Mougel M, Blanchard JM, Sitbon M, Bertrand E (2003) Retroviral genomic RNAs are transported to the plasma membrane by endosomal vesicles. Dev Cell 5: 161–174 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

