Coordinated action of NSF and PKC regulates GABAB receptor signaling efficacy
- PMID: 16724110
- PMCID: PMC1500845
- DOI: 10.1038/sj.emboj.7601157
Coordinated action of NSF and PKC regulates GABAB receptor signaling efficacy
Abstract
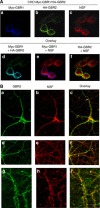
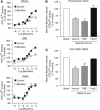
The obligatory heterodimerization of the GABAB receptor (GBR) raises fundamental questions about molecular mechanisms controlling its signaling efficacy. Here, we show that NEM sensitive fusion (NSF) protein interacts directly with the GBR heterodimer both in rat brain synaptosomes and in CHO cells, forming a ternary complex that can be regulated by agonist stimulation. Inhibition of NSF binding with a peptide derived from GBR2 (TAT-Pep-27) did not affect basal signaling activity but almost completely abolished agonist-promoted GBR desensitization in both CHO cells and hippocampal slices. Taken with the role of PKC in the desensitization process, our observation that TAT-Pep-27 prevented both agonist-promoted recruitment of PKC and receptor phosphorylation suggests that NSF is a priming factor required for GBR desensitization. Given that GBR desensitization does not involve receptor internalization, the NSF/PKC coordinated action revealed herein suggests that NSF can regulate GPCR signalling efficacy independently of its role in membrane trafficking. The functional interaction between three bona fide regulators of neurotransmitter release, such as GBR, NSF and PKC, could shed new light on the modulation of presynaptic GBR action.
Figures








References
-
- Barclay JW, Craig TJ, Fisher RJ, Ciufo LF, Evans GJ, Morgan A, Burgoyne RD (2003) Phosphorylation of Munc18 by protein kinase C regulates the kinetics of exocytosis. J Biol Chem 278: 10538–10545 - PubMed
-
- Becher A, Green A, Ige AO, Wise A, White JH, McIlhinney RA (2004) Ectopically expressed gamma-aminobutyric acid receptor B is functionally down-regulated in isolated lipid raft-enriched membranes. Biochem Biophys Res Commun 321: 981–987 - PubMed
-
- Becker-Hapak M, McAllister SS, Dowdy SF (2001) TAT-mediated protein transduction into mammalian cells. Methods 24: 247–256 - PubMed
-
- Blumberg PM (1991) Complexities of the protein kinase C pathway. Mol Carcinog 4: 339–344 - PubMed
-
- Bomberger JM, Parameswaran N, Hall CS, Aiyar N, Spielman WS (2005) Novel function for receptor activity-modifying proteins (RAMPs) in post-endocytic receptor trafficking. J Biol Chem 280: 9297–9307 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

