Induction of antitumor immunity through xenoplacental immunization
- PMID: 16725035
- PMCID: PMC1482718
- DOI: 10.1186/1479-5876-4-22
Induction of antitumor immunity through xenoplacental immunization
Abstract
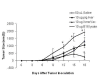
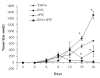
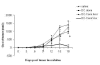
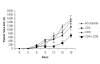
Historically cancer vaccines have yielded suboptimal clinical results. We have developed a novel strategy for eliciting antitumor immunity based upon homology between neoplastic tissue and the developing placenta. Placenta formation shares several key processes with neoplasia, namely: angiogenesis, activation of matrix metalloproteases, and active suppression of immune function. Immune responses against xenoantigens are well known to break self-tolerance. Utilizing xenogeneic placental protein extracts as a vaccine, we have successfully induced anti-tumor immunity against B16 melanoma in C57/BL6 mice, whereas control xenogeneic extracts and B16 tumor extracts where ineffective, or actually promoted tumor growth, respectively. Furthermore, dendritic cells were able to prime tumor immunity when pulsed with the placental xenoantigens. While vaccination-induced tumor regression was abolished in mice depleted of CD4 T cells, both CD4 and CD8 cells were needed to adoptively transfer immunity to naïve mice. Supporting the role of CD8 cells in controlling tumor growth are findings that only freshly isolated CD8 cells from immunized mice were capable of inducing tumor cell caspases-3 activation ex vivo. These data suggest feasibility of using xenogeneic placental preparations as a multivalent vaccine potently targeting not just tumor antigens, but processes that are essential for tumor maintenance of malignant potential.
Figures
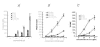
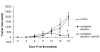
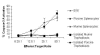
References
-
- Pijnenborg R. Implantation and immunology: maternal inflammatory and immune cellular responses to implantation and trophoblast invasion. Reprod Biomed Online. 2002;4 Suppl 3:14–17. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

