Beginning at the end: repetitive firing properties in the final common pathway
- PMID: 16725251
- PMCID: PMC5061565
- DOI: 10.1016/j.pneurobio.2006.04.002
Beginning at the end: repetitive firing properties in the final common pathway
Abstract
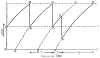
Since the early 20th century, it has been recognized that motoneurons must fire repetitive trains of action potentials to produce muscle contraction. In 1932, Sir John Eccles, together with Hebbel Hoff, found that action potential spike trains in motor axons were produced by "rhythmic centres", which were within the motoneurons themselves. Two decades later, Eccles attended a Cold Spring Harbor Symposium in NY, USA entitled "The Neuron". Two of the many notable presentations at this symposium were juxtaposed: one by Eccles from the University of Otago, Dunedin, NZL, and the other by J. Walter Woodbury and Harry Patton from the University of Washington, Seattle, USA. Both presentations included data obtained using sharp microelectrodes to study the intracellularly recorded potentials of cat motoneurons. In this review, I discuss some of the events leading up to and surrounding this jointly accomplished advance and proceed to discussion of subsequent studies over 5+ decades that have made use of intracellular recordings from motoneurons to study their repetitive firing behavior. This begins with early descriptions of primary and secondary range firing, and continues to the discovery of dendritic persistent inward currents and their relation to plateau potentials, synaptic amplification, and motoneuronal firing. Following a brief description of the possible mechanisms underlying spike frequency adaptation, I discuss the modulation of repetitive firing properties during various motor behaviors. It has become increasingly clear that the central nervous system has exquisite control of the repetitive firing of motoneurons. Eccles' work laid the foundation for the present-day study of these processes.
Figures

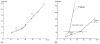

References
-
- Alvarez FJ, Pearson JC, Harrington D, Dewey D, Torbeck L, Fyffe RE. Distribution of 5-hydroxytryptamine-immunoreactive boutons on alpha-motoneurons in the lumbar spinal cord of adult cats. J Comp Neurol. 1998;393:69–83. - PubMed
-
- Andersson O, Forssberg H, Grillner S, Lindquist M. Phasic gain control of the transmission in cutaneous reflex pathways to motoneurons during ‘fictive’ locomotion. Brain Res. 1978;149:503–507. - PubMed
-
- Araki T, Otani T. Response of single motoneurons to direct stimulation in toad’s spinal cord. J Neurophysiol. 1955;18:472–485. - PubMed
-
- Baldissera F, Gustafsson B. Regulation of repetitive firing in motoneurons by the afterhyperpolarization conductance. Brain Res. 1971a;30:431–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

