Cellular inhibitors of long interspersed element 1 and Alu retrotransposition
- PMID: 16728505
- PMCID: PMC1482655
- DOI: 10.1073/pnas.0603313103
Cellular inhibitors of long interspersed element 1 and Alu retrotransposition
Abstract
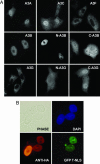
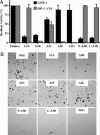
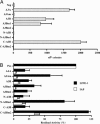
Long interspersed element (LINE) 1 retrotransposons are major genomic parasites that represent approximately 17% of the human genome. The LINE-1 ORF2 protein is also responsible for the mobility of Alu elements, which constitute a further approximately 11% of genomic DNA. Representative members of each element class remain mobile, and deleterious retrotransposition events can induce spontaneous genetic diseases. Here, we demonstrate that APOBEC3A and APOBEC3B, two members of the APOBEC3 family of human innate antiretroviral resistance factors, can enter the nucleus, where LINE-1 and Alu reverse transcription occurs, and specifically inhibit both LINE-1 and Alu retrotransposition. These data suggest that the APOBEC3 protein family may have evolved, at least in part, to defend the integrity of the human genome against endogenous retrotransposons.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


References
-
- Moran J. V., Gilbert N. In: Mobile DNA II. Craig N., Craggie R., Gellert M., Lambowitz A., editors. Washington, DC: Am. Soc. Microbiol.; 2002. pp. 836–869.
-
- Moran J. V., Holmes S. E., Naas T. P., DeBerardinis R. J., Boeke J. D., Kazazian H. H., Jr Cell. 1996;87:917–927. - PubMed
-
- Batzer M. A., Deininger P. L. Nat. Rev. Genet. 2002;3:370–379. - PubMed
-
- Dewannieux M., Esnault C., Heidmann T. Nat. Genet. 2003;35:41–48. - PubMed
-
- Lander E. S., Linton L. M., Birren B., Nusbaum C., Zody M. C., Baldwin J., Devon K., Dewar K., Doyle M., FitzHugh W., et al. Nature. 2001;409:860–921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

