A coherent feed-forward loop with a SUM input function prolongs flagella expression in Escherichia coli
- PMID: 16729041
- PMCID: PMC1681456
- DOI: 10.1038/msb4100010
A coherent feed-forward loop with a SUM input function prolongs flagella expression in Escherichia coli
Abstract
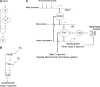
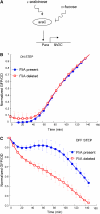
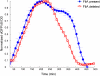
Complex gene-regulation networks are made of simple recurring gene circuits called network motifs. The functions of several network motifs have recently been studied experimentally, including the coherent feed-forward loop (FFL) with an AND input function that acts as a sign-sensitive delay element. Here, we study the function of the coherent FFL with a sum input function (SUM-FFL). We analyze the dynamics of this motif by means of high-resolution expression measurements in the flagella gene-regulation network, the system that allows Escherichia coli to swim. In this system, the master regulator FlhDC activates a second regulator, FliA, and both activate in an additive fashion the operons that produce the flagella motor. We find that this motif prolongs flagella expression following deactivation of the master regulator, protecting flagella production from transient loss of input signal. Thus, in contrast to the AND-FFL that shows a delay following signal activation, the SUM-FFL shows delays after signal deactivation. The SUM-FFL in this system works as theoretically predicted despite being embedded in at least two additional feedback loops. The present function might be carried out by the SUM-FFL in systems found across organisms.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

