Phosphotyrosine interactome of the ErbB-receptor kinase family
- PMID: 16729043
- PMCID: PMC1681463
- DOI: 10.1038/msb4100012
Phosphotyrosine interactome of the ErbB-receptor kinase family
Abstract
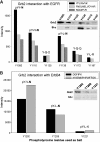
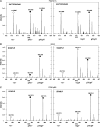
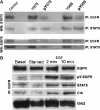
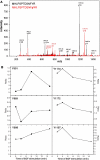
Interactions between short modified peptide motifs and modular protein domains are central events in cell signal-transduction. We determined interaction partners to all cytosolic tyrosine residues of the four members of the ErbB-receptor family in an unbiased fashion by quantitative proteomics using pull-down experiments with pairs of phosphorylated and nonphosphorylated synthetic peptides. Each receptor had characteristic preferences for interacting proteins and most interaction partners had multiple binding sites on each receptor. EGFR and ErbB4 had several docking sites for Grb2, while ErbB3 was characterized by six binding sites for PI3K. We identified STAT5 as a direct binding partner to EGFR and ErbB4 and discovered new recognition motifs for Shc and STAT5. The overall pattern of interaction partners of EGFR and ErbB4 suggests similar roles during signaling through their respective ligands. Phosphorylation kinetics of several tyrosine resides was measured by mass spectrometry and correlated with interaction partner preference. Our results demonstrate that system-wide mapping of peptide-protein interactions sites is possible, and suggest shared and unique roles of ErbB-receptor family members in downstream signaling.
Figures


References
-
- Andersen JS, Wilkinson CJ, Myoru T, Mortensen P, Nigg EA, Mann M (2003) Proteomic characterization of the human centrosome by protein correlation profiling. Nature 426: 570–574 - PubMed
-
- Birge RB, Fajardo JE, Mayer BJ, Hanafusa H (1992) Tyrosine-phosphorylated epidermal growth factor receptor and cellular p130 provide high affinity binding substrates to analyze Crk-phosphotyrosine-dependent interactions in vitro. J Biol Chem 267: 10588–10595 - PubMed
-
- Blagoev B, Kratchmarova I, Ong S-E, Nielsen M, Foster L, Mann M (2003) A proteomics strategy to elucidate functional protein-protein interactions applied to EGF signaling. Nat Biotechnol 21: 315–318 - PubMed
-
- Blagoev B, Ong S-E, Kratchmarova I, Mann M (2004) Temporal ordering of signaling networks by quantitive proteomics. Nat Biotechnol 22: 1139–1145 - PubMed
-
- Blume-Jensen P, Hunter T (2001) Oncogenic kinase signalling. Nature 411: 355–365 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

