Paramagnetic lanthanide complexes as PARACEST agents for medical imaging
- PMID: 16729144
- PMCID: PMC2718840
- DOI: 10.1039/b509907m
Paramagnetic lanthanide complexes as PARACEST agents for medical imaging
Abstract
This tutorial review examines the fundamental aspects of a new class of contrast media for MRI based upon the chemical shift saturation transfer (CEST) mechanism. Several paramagnetic versions called PARACEST agents have shown utility as responsive agents for reporting physiological or metabolic information by MRI. It is shown that basic NMR exchange theory can be used to predict how parameters such as chemical shift, bound water lifetimes, and relaxation rates can be optimized to maximize the sensitivity of PARACEST agents.
Figures
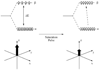

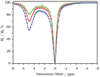

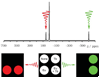
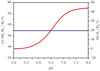
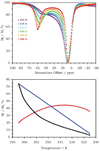
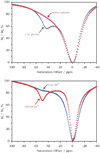
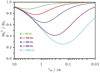

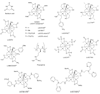
References
-
- Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem. Rev. 1999;99:2293. and references cited therein. - PubMed
-
- Sanders JKM, Hunter BK. Modern NMR Spectroscopy; A Guide for Chemists. Oxford, UK: Oxford University Press; 1997. and references cited therein.
-
- Henkelman RM, Stanisz GJ, Graham SJ. NMR Biomed. 2001;14:57. and references cited therein. - PubMed
-
- Ward KM, Aletras AH, Balaban RS. J. Magn. Reson. 2000;143:79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

