Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes
- PMID: 16731586
- PMCID: PMC1488921
- DOI: 10.1105/tpc.105.039859
Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes
Abstract
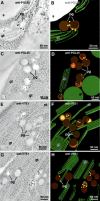
Plastoglobules are lipoprotein particles inside chloroplasts. Their numbers have been shown to increase during the upregulation of plastid lipid metabolism in response to oxidative stress and during senescence. In this study, we used state-of-the-art high-pressure freezing/freeze-substitution methods combined with electron tomography as well as freeze-etch electron microscopy to characterize the structure and spatial relationship of plastoglobules to thylakoid membranes in developing, mature, and senescing chloroplasts. We demonstrate that plastoglobules are attached to thylakoids through a half-lipid bilayer that surrounds the globule contents and is continuous with the stroma-side leaflet of the thylakoid membrane. During oxidative stress and senescence, plastoglobules form linkage groups that are attached to each other and remain continuous with the thylakoid membrane by extensions of the half-lipid bilayer. Using three-dimensional tomography combined with immunolabeling techniques, we show that the plastoglobules contain the enzyme tocopherol cyclase (VTE1) and that this enzyme extends across the surface monolayer into the interior of the plastoglobules. These findings demonstrate that plastoglobules function as both lipid biosynthesis and storage subcompartments of thylakoid membranes. The permanent structural coupling between plastoglobules and thylakoid membranes suggests that the lipid molecules contained in the plastoglobule cores (carotenoids, plastoquinone, and tocopherol [vitamin E]) are in a dynamic equilibrium with those located in the thylakoid membranes.
Figures







References
-
- Austin, J.R., Segui-Simarro, J.M., and Staehelin, L.A. (2005). Quantitative analysis of changes in spatial distribution and plus-end geometry of microtubules involved in plant-cell cytokinesis. J. Cell Sci. 118 3895–3903. - PubMed
-
- Bathgate, B., Purton, M.E., Grierson, D., and Goodenough, P.W. (1985). Plastid changes during the conversion of chloroplasts to chromoplasts in ripening tomatoes. Planta 165 197–204. - PubMed
-
- Britvec, M., Reichenauer, T., Soja, G., Ljubesic, N., Eid, M., and Pecina, M. (2001). Ultrastructure changes in grapevine chloroplasts caused by increased tropospheric ozone concentrations. Biologia (Bratisl.) 56 417–424.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

