Trimethylation of histone H3 lysine 4 by Set1 in the lytic infection of human herpes simplex virus 1
- PMID: 16731913
- PMCID: PMC1472580
- DOI: 10.1128/JVI.00169-06
Trimethylation of histone H3 lysine 4 by Set1 in the lytic infection of human herpes simplex virus 1
Abstract
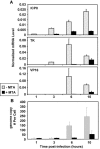
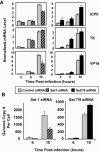
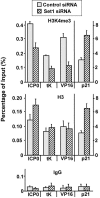
Human herpes simplex virus 1 (HSV-1) is a double-stranded DNA virus that causes facial, ocular, and encephalitic disease in humans. Previous work showed that the genome of HSV-1 is associated with acetylated and methylated histones during lytic infection. However, the physiological role of histone modifications in lytic infection of HSV-1 is unclear. We examined the role of protein methylation in lytic infection of HSV-1 using a protein methylation inhibitor, 5'-deoxy-5'-methylthioadenosine (MTA). We found that MTA strongly reduces the transcription and replication of HSV-1. Moreover, MTA treatment decreases the level of trimethylation of lysine 4 in histone H3 (H3K4me3) on the HSV-1 genome. These results suggest that protein methylation, and in particular, histone methylation, is involved in the lytic infection of HSV-1. To delineate the underlying mechanism, we investigated the role of two H3K4 methyltransferases, Set1 and Set7/9, in the lytic infection of HSV-1. Using small interference RNA, we found that the reduction of Set1, but not Set7/9, reduces the transcription and replication of HSV-1 and specifically decreases H3K4me3 on the virus genome. These results indicate that H3K4me3 mediated by Set1 is required for optimal gene expression and replication of HSV-1 during lytic infection and suggest that this pathway could be a potential point of pharmacological intervention during HSV-1 infection.
Figures

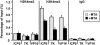


References
-
- Avila, M. A., E. R. Garcia-Trevijano, S. C. Lu, F. J. Corrales, and J. M. Mato. Methylthioadenosine. Int. J. Biochem. Cell Biol. 36:2125-2130. - PubMed
-
- Bannister, A. J., and T. Kouzarides. 1996. The CBP co-activator is a histone acetyltransferase. Nature 384:641-643. - PubMed
-
- Bannister, A. J., P. Zegerman, J. F. Partridge, E. A. Miska, J. O. Thomas, R. C. Allshire, and T. Kouzarides. 2001. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410:120. - PubMed
-
- Berger, S. L. 2002. Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev. 12:142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

