Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex
- PMID: 16731931
- PMCID: PMC1472606
- DOI: 10.1128/JVI.02501-05
Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex
Abstract
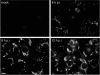
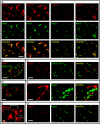
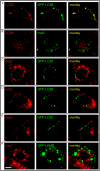
The RNA replication complexes of mammalian positive-stranded RNA viruses are generally associated with (modified) intracellular membranes, a feature thought to be important for creating an environment suitable for viral RNA synthesis, recruitment of host components, and possibly evasion of host defense mechanisms. Here, using a panel of replicase-specific antisera, we have analyzed the earlier stages of severe acute respiratory syndrome coronavirus (SARS-CoV) infection in Vero E6 cells, in particular focusing on the subcellular localization of the replicase and the ultrastructure of the associated membranes. Confocal immunofluorescence microscopy demonstrated the colocalization, throughout infection, of replicase cleavage products containing different key enzymes for SARS-CoV replication. Electron microscopy revealed the early formation and accumulation of typical double-membrane vesicles, which probably carry the viral replication complex. The vesicles appear to be fragile, and their preservation was significantly improved by using cryofixation protocols and freeze substitution methods. In immunoelectron microscopy, the virus-induced vesicles could be labeled with replicase-specific antibodies. Opposite to what was described for mouse hepatitis virus, we did not observe the late relocalization of specific replicase subunits to the presumed site of virus assembly, which was labeled using an antiserum against the viral membrane protein. This conclusion was further supported using organelle-specific marker proteins and electron microscopy. Similar morphological studies and labeling experiments argued against the previously proposed involvement of the autophagic pathway as the source for the vesicles with which the replicase is associated and instead suggested the endoplasmic reticulum to be the most likely donor of the membranes that carry the SARS-CoV replication complex.
Figures




References
-
- Anand, K., J. Ziebuhr, P. Wadhwani, J. R. Mesters, and R. Hilgenfeld. 2003. Coronavirus main proteinase (3CL(pro)) structure: basis for design of anti-SARS drugs. Science 300:1763-1767. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

