Neutralizing antibody responses against autologous and heterologous viruses in acute versus chronic human immunodeficiency virus (HIV) infection: evidence for a constraint on the ability of HIV to completely evade neutralizing antibody responses
- PMID: 16731954
- PMCID: PMC1472617
- DOI: 10.1128/JVI.00093-06
Neutralizing antibody responses against autologous and heterologous viruses in acute versus chronic human immunodeficiency virus (HIV) infection: evidence for a constraint on the ability of HIV to completely evade neutralizing antibody responses
Abstract
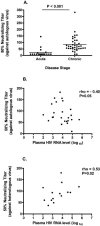
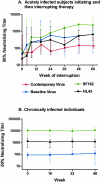
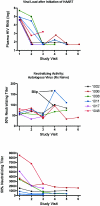
Acute human immunodeficiency virus (HIV) infection is associated with the rapid development of neutralization escape mutations. The degree to which viral evolution persists in chronic infection has not been well characterized, nor is it clear if all patients develop high-level neutralization antibody escape. We therefore measured neutralizing antibody responses against autologous and heterologous viruses in a cohort of acutely and chronically infected subjects (n = 65). Neutralizing antibody responses against both autologous virus and heterologous viruses were lower among individuals with acute infection than among those with chronic infection. Among chronically infected individuals, there was a negative correlation between the level of neutralizing antibodies against autologous virus and the level of viremia. In contrast, there was a positive correlation between the level of neutralizing antibodies against a panel of heterologous viruses and the level of viremia. Viral evolution, as defined by the presence of higher neutralizing titers directed against earlier viruses than against contemporaneous viruses, was evident for subjects with recent infection but absent for those with chronic infection. In summary, neutralizing antibody responses against contemporaneous autologous viruses are absent in early HIV infection but can be detected at low levels in chronic infection, particularly among those controlling HIV in the absence of therapy. HIV replication either directly or indirectly drives the production of increasing levels of antibodies that cross-neutralize heterologous primary isolates. Collectively, these observations indicate that although HIV continuously drives the production of neutralizing antibodies, there may be limits to the capacity of the virus to evolve continuously in response to these antibodies. These observations also suggest that the neutralizing antibody response may contribute to the long-term control of HIV in some patients while protecting against HIV superinfection in most patients.
Figures
References
-
- Alatrakchi, N., C. Duvivier, D. Costagliola, A. Samri, A. Marcelin, G. Kamkamidze, M. Astriti, R. Agher, V. Calvez, B. Autran, and C. Katlama. 2005. Persistent low viral load on antiretroviral therapy is associated with T cell-mediated control of HIV replication. AIDS 19:25-33. - PubMed
-
- Albert, J., B. Abrahamsson, K. Nagy, E. Aurelius, H. Gaines, G. Nystrom, and E. M. Fenyo. 1990. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS 4:107-112. - PubMed
-
- Altfeld, M., T. M. Allen, X. G. Yu, M. N. Johnston, D. Agrawal, B. T. Korber, D. C. Montefiori, D. H. O'Connor, B. T. Davis, P. K. Lee, E. L. Maier, J. Harlow, P. J. Goulder, C. Brander, E. S. Rosenberg, and B. D. Walker. 2002. HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus. Nature 420:434-439. - PubMed
-
- Barbour, J. D., T. Wrin, R. M. Grant, J. N. Martin, M. R. Segal, C. J. Petropoulos, and S. G. Deeks. 2002. Evolution of phenotypic drug susceptibility and viral replication capacity during long-term virologic failure of protease inhibitor therapy in human immunodeficiency virus-infected adults. J. Virol. 76:11104-11112. - PMC - PubMed
-
- Barker, E., C. E. Mackewicz, G. Reyes-Teran, A. Sato, S. A. Stranford, S. H. Fujimura, C. Christopherson, S. Y. Chang, and J. A. Levy. 1998. Virological and immunological features of long-term human immunodeficiency virus-infected individuals who have remained asymptomatic compared with those who have progressed to acquired immunodeficiency syndrome. Blood 92:3105-3114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 MH062246/MH/NIMH NIH HHS/United States
- 5-M01-RR00083-37/RR/NCRR NIH HHS/United States
- AI47062/AI/NIAID NIH HHS/United States
- R21 AI055273/AI/NIAID NIH HHS/United States
- R01 AI044595/AI/NIAID NIH HHS/United States
- R01 AI052745/AI/NIAID NIH HHS/United States
- P30 MH59037/MH/NIMH NIH HHS/United States
- AI44595/AI/NIAID NIH HHS/United States
- AI055273/AI/NIAID NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- M01 RR000083/RR/NCRR NIH HHS/United States
- P30 MH62246/MH/NIMH NIH HHS/United States
- R01 AI047062/AI/NIAID NIH HHS/United States
- P30 AI27763/AI/NIAID NIH HHS/United States
- AI052745/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

