Transcriptional silencing of multiple genes in trophozoites of Entamoeba histolytica
- PMID: 16733544
- PMCID: PMC1464398
- DOI: 10.1371/journal.ppat.0020048
Transcriptional silencing of multiple genes in trophozoites of Entamoeba histolytica
Abstract
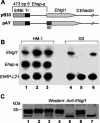
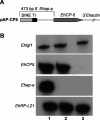
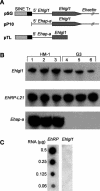
In a previous work we described the transcriptional silencing of the amoebapore A (AP-A) gene (Ehap-a) of Entamoeba histolytica strain HM-1:IMSS. The silencing occurred following transfection with a plasmid containing a 5' upstream region (473 bp) of Ehap-a that included a truncated segment (140 bp) of a short interspersed nuclear element (SINE1). Silencing remained in effect even after removal of the plasmid (clone G3). Neither short interfering RNA nor methylated DNA were detected, but the chromatin domain of Ehap-a in the gene-silenced trophozoites was modified. Two other similar genes (Ehap-b and one encoding a Saposin-like protein, SAPLIP 1) also became silenced. In the present work we demonstrate the silencing of a second gene of choice, one that encodes the light subunit of the Gal/GalNAc inhibitable lectin (Ehlgl1) and the other, the cysteine proteinase 5 (EhCP-5). This silencing occurred in G3 trophozoites transfected with a plasmid in which the 473 bp 5' upstream Ehap-a fragment was directly ligated to the second gene. Transcriptional silencing occurred in both the transgene and the chromosomal gene. SINE1 sequences were essential, as was a direct connection between the Ehap-a upstream region and the beginning of the open reading frame of the second gene. Gene silencing did not occur in strain HM-1:IMSS with any of these plasmid constructs. The trophozoites with two silenced genes were virulence-attenuated as were those of clone G3. In addition, trophozoites not expressing Lgl1 and AP-A proteins had a significantly reduced ability to cap the Gal/GalNAc-lectin to the uroid region when incubated with antibodies against the heavy (170 kDa) subunit of the lectin. Lysates of trophozoites lacking cysteine proteinase 5 and AP-A proteins had 30% less cysteine proteinase activity than those of HM-1:IMSS strain or the G3 clone. Silencing of other genes in G3 amoebae could provide a model to study their various functions. In addition, double gene-silenced, virulence-attenuated trophozoites may be an important tool in vaccine development.
Conflict of interest statement
Figures





References
-
- Baulcombe D. RNA silencing in plants. Nature. 2004;431:356–363. - PubMed
-
- Cogoni C. Homology-dependent gene silencing mechanisms in fungi. Annu Rev Microbiol. 2001;55:381–406. - PubMed
-
- Hannon GJ. RNA interference. Nature. 2002;418:244–251. - PubMed
-
- Moazed D. Common themes in mechanisms of gene silencing. Mol Cell. 2001;8:489–498. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

