Changes in gene expression foreshadow diet-induced obesity in genetically identical mice
- PMID: 16733553
- PMCID: PMC1464831
- DOI: 10.1371/journal.pgen.0020081
Changes in gene expression foreshadow diet-induced obesity in genetically identical mice
Abstract
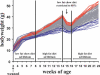
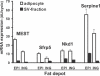
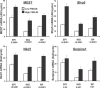
High phenotypic variation in diet-induced obesity in male C57BL/6J inbred mice suggests a molecular model to investigate non-genetic mechanisms of obesity. Feeding mice a high-fat diet beginning at 8 wk of age resulted in a 4-fold difference in adiposity. The phenotypes of mice characteristic of high or low gainers were evident by 6 wk of age, when mice were still on a low-fat diet; they were amplified after being switched to the high-fat diet and persisted even after the obesogenic protocol was interrupted with a calorically restricted, low-fat chow diet. Accordingly, susceptibility to diet-induced obesity in genetically identical mice is a stable phenotype that can be detected in mice shortly after weaning. Chronologically, differences in adiposity preceded those of feeding efficiency and food intake, suggesting that observed difference in leptin secretion is a factor in determining phenotypes related to food intake. Gene expression analyses of adipose tissue and hypothalamus from mice with low and high weight gain, by microarray and qRT-PCR, showed major changes in the expression of genes of Wnt signaling and tissue re-modeling in adipose tissue. In particular, elevated expression of SFRP5, an inhibitor of Wnt signaling, the imprinted gene MEST and BMP3 may be causally linked to fat mass expansion, since differences in gene expression observed in biopsies of epididymal fat at 7 wk of age (before the high-fat diet) correlated with adiposity after 8 wk on a high-fat diet. We propose that C57BL/6J mice have the phenotypic characteristics suitable for a model to investigate epigenetic mechanisms within adipose tissue that underlie diet-induced obesity.
Conflict of interest statement
Competing interests. Dr. Jihad S. Skaf is a paid employee of Applied Biosystems Inc.; his job title is Technical Applications Specialist.
Figures

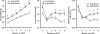


References
-
- Bouchard C, Tremblay A, Despres JP, Nadeau A, Lupien PJ, et al. The response to long-term overfeeding in identical twins. N Engl J Med. 1990;322:1477–1482. - PubMed
-
- Allison DB, Kaprio J, Korkeila M, Koskenvuo M, Neale MC, et al. The heritability of body mass index among an international sample of monozygotic twins reared apart. Int J Obes Relat Metab Disord. 1996;20:501–506. - PubMed
-
- Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997;27:325–351. - PubMed
-
- Perusse L, Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, et al. The human obesity gene map: The 2004 update. Obes Res. 2005;13:381–490. - PubMed
-
- Stunkard AJ, Harris JR, Pedersen NL, McClearn GE. The body-mass index of twins who have been reared apart. N Engl J Med. 1990;322:1483–1487. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

