In vivo tracking of macrophage activated killer cells to sites of metastatic ovarian carcinoma
- PMID: 16733671
- PMCID: PMC11030026
- DOI: 10.1007/s00262-006-0181-3
In vivo tracking of macrophage activated killer cells to sites of metastatic ovarian carcinoma
Abstract
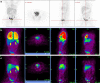
Radio-labelling of blood cells is an established technique for evaluating in vivo migration of normal cells to sites of pathology such as infection and haemorrhage. A limitation of cellular immunotherapies to induce anti-tumour responses is in part due to the uncertain ability of cellular effectors to reach their intended target. We extended the approach of cell radiolabelling to accurately examine the in vivo distribution of cellular immunotherapy with ex-vivo macrophage activated killer (MAK) cells. We describe the use of two methods of cell labelling for tracking the destination of autologous-derived macrophage activated killer (MAK) cells linked to the bi-specific antibody MDX-H210 delivered either by intravenous (i.v.) or intraperitoneal (i.p.) injection in ten patients with peritoneal relapse of epithelial ovarian carcinoma. Our results demonstrate the feasibility of generating high numbers and purity of GMP quality MAK cells, which can be radiolabelled with (18)F-FDG or (111)In-oxime. MAK cell administration produced minimal infusional toxicity and demonstrated a reproducible pattern of in vivo distribution and active in vivo tracking to sites of known tumour following 8 of 16 i.v. infusions or 4 of 6 i.p. infusions. However, the leakage of (18)F-FDG limited the ability to confidently confirm the tracking of MAK cells to tumour in all cases and improved PET labels are required. The addition of MDX-H210 bispecific antibody did not alter the distribution of cells to tumour sites, but did accelerate the clearance of i.v. administered MAK cells from the pulmonary circulation. This data demonstrates that cellular cancer immunotherapies may be successfully delivered to the sites of active tumour following either i.v. or i.p. injection in a proportion of patients with metastatic cancer. Incorporation of tracking studies in early cycles of cellular immunotherapy may allow selection of patients who demonstrate successful targeting of the immunotherapy for ongoing treatment.
Figures



References
-
- Chokri M, Lopez M, Oleron C, Girard A, Martinache C, Siffert JC, Canepa S, Bartholeyns J. Production of macrophages with potent antitumoral properties (MAK) by culture of monocytes in the presence of GM-CSF and 1,25 (OH)2 vitD3. Anticancer Res. 1992;12:2257–2260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

