CD28 co-stimulation via tumour-specific chimaeric receptors induces an incomplete activation response in Epstein-Barr virus-specific effector memory T cells
- PMID: 16734614
- PMCID: PMC1941988
- DOI: 10.1111/j.1365-2249.2006.03095.x
CD28 co-stimulation via tumour-specific chimaeric receptors induces an incomplete activation response in Epstein-Barr virus-specific effector memory T cells
Abstract
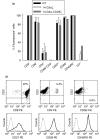
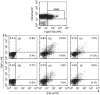
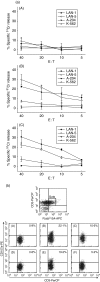
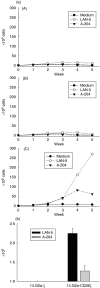
Expression of tumour antigen-specific chimaeric receptors in T lymphocytes can redirect their effector functions towards tumour cells. Integration of the signalling domains of the co-stimulatory molecule CD28 into chRec enhances antigen-specific proliferation of polyclonal human T cell populations. While CD28 plays an essential role in the priming of naive CD4(+) T cells, its contribution to effector memory T cell responses is controversial. We compared the function of the chRec with and without the CD28 co-stimulatory domain, expressing it in peripheral blood T cells or Epstein-Barr virus (EBV)-specific T cell lines. The chimaeric T cell receptors contain an extracellular single-chain antibody domain, to give specificity against the tumour ganglioside antigen G(D2). The transduced cytotoxic T lymphocytes (CTL) maintained their specificity for autologous EBV targets and their capacity to proliferate after stimulation with EBV-infected B cells. Intracellular cytokine staining demonstrated efficient and comparable antigen-specific interferon (IFN)-gamma secretion by CTL following engagement of both the native and the chimaeric receptor, independent of chimaeric CD28 signalling. Furthermore, tumour targets were lysed in an antigen-specific manner by both chRec. However, while antigen engagement by CD28 zeta chRec efficiently induced expansion of polyclonal peripheral blood lymphocytes in an antigen-dependent manner, CD28 signalling did not induce proliferation of EBV-CTL in response to antigen-expressing tumour cells. Thus, the co-stimulatory requirement for the efficient activation response of antigen-specific memory cells cannot be mimicked simply by combining CD28 and zeta signalling. The full potential of this highly cytolytic T cell population for adoptive immunotherapy of cancer requires further exploration of their co-stimulatory requirements.
Figures
References
-
- Rossig C, Brenner MK. Genetic modification of T lymphocytes for adoptive immunotherapy. Mol Ther. 2004;10:5–18. - PubMed
-
- Brentjens RJ, Latouche JB, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

