Polarized axonal surface expression of neuronal KCNQ channels is mediated by multiple signals in the KCNQ2 and KCNQ3 C-terminal domains
- PMID: 16735477
- PMCID: PMC1472242
- DOI: 10.1073/pnas.0603376103
Polarized axonal surface expression of neuronal KCNQ channels is mediated by multiple signals in the KCNQ2 and KCNQ3 C-terminal domains
Abstract
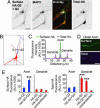
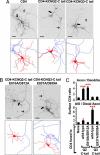
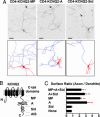
The M channels, important regulators of neuronal excitability, are voltage-gated potassium channels composed of KCNQ2-5 subunits. Mutations in KCNQ2 and KCNQ3 cause benign familial neonatal convulsions (BFNC), dominantly inherited epilepsy and myokymia. Crucial for their functions in controlling neuronal excitability, the M channels must be placed at specific regions of the neuronal membrane. However, the precise distribution of surface KCNQ channels is not known. Here, we show that KCNQ2/KCNQ3 channels are preferentially localized to the surface of axons both at the axonal initial segment and more distally. Whereas axonal initial segment targeting of surface KCNQ channels is mediated by ankyrin-G binding motifs of KCNQ2 and KCNQ3, sequences mediating targeting to more distal portion of the axon reside in the membrane proximal and A domains of the KCNQ2 C-terminal tail. We further show that several BFNC mutations of KCNQ2 and KCNQ3 disrupt surface expression or polarized surface distribution of KCNQ channels, thereby revealing impaired targeting of KCNQ channels to axonal surfaces as a BFNC etiology.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

