Cysteine biosynthesis in Trichomonas vaginalis involves cysteine synthase utilizing O-phosphoserine
- PMID: 16735516
- PMCID: PMC2645516
- DOI: 10.1074/jbc.M600688200
Cysteine biosynthesis in Trichomonas vaginalis involves cysteine synthase utilizing O-phosphoserine
Abstract
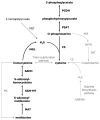
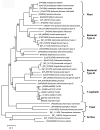
Trichomonas vaginalis is an early divergent eukaryote with many unusual biochemical features. It is an anaerobic protozoan parasite of humans that is thought to rely heavily on cysteine as a major redox buffer, because it lacks glutathione. We report here that for synthesis of cysteine from sulfide, T. vaginalis relies upon cysteine synthase. The enzyme (TvCS1) can use either O-acetylserine or O-phosphoserine as substrates. The K(m) values of the enzyme for sulfide are very low (0.02 mm), suggesting that the enzyme may be a means of ensuring that sulfide in the parasite is maintained at a low level. T. vaginalis appears to lack serine acetyltransferase, the source of O-acetylserine in many cells, but has a functional 3-phosphoglycerate dehydrogenase and an O-phosphoserine aminotransferase that together result in the production of O-phosphoserine, suggesting that this is the physiological substrate. TvCS1 can also use thiosulfate as substrate. Overall, TvCS1 has substrate specificities similar to those reported for cysteine synthases of Aeropyrum pernix and Escherichia coli, and this is reflected by sequence similarities around the active site. We suggest that these enzymes are classified together as type B cysteine synthases, and we hypothesize that the use of O-phosphoserine is a common characteristic of these cysteine synthases. The level of cysteine synthase in T. vaginalis is regulated according to need, such that parasites growing in an environment rich in cysteine have low activity, whereas exposure to propargylglycine results in elevated cysteine synthase activity. Humans lack cysteine synthase; therefore, this parasite enzyme could be an exploitable drug target.
Figures



References
-
- World Health Organisation Global prevalence and incidence of selected curable sexually transmitted diseases: overview and estimates. 2001 WHO/CDS/CSR/EDC/2001.10.
-
- Baldauf SL, Roger AJ, Wenk-Siefert I, Doolittle WF. Science. 2000;290:972–977. - PubMed
-
- Horner DS, Embley TM. Mol. Biol. Evol. 2001;18:1970–1975. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

