An intimate collaboration between peroxisomes and lipid bodies
- PMID: 16735577
- PMCID: PMC2063889
- DOI: 10.1083/jcb.200511125
An intimate collaboration between peroxisomes and lipid bodies
Abstract
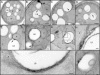
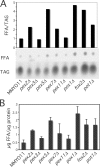
Although peroxisomes oxidize lipids, the metabolism of lipid bodies and peroxisomes is thought to be largely uncoupled from one another. In this study, using oleic acid-cultured Saccharomyces cerevisiae as a model system, we provide evidence that lipid bodies and peroxisomes have a close physiological relationship. Peroxisomes adhere stably to lipid bodies, and they can even extend processes into lipid body cores. Biochemical experiments and proteomic analysis of the purified lipid bodies suggest that these processes are limited to enzymes of fatty acid beta oxidation. Peroxisomes that are unable to oxidize fatty acids promote novel structures within lipid bodies ("gnarls"), which may be organized arrays of accumulated free fatty acids. However, gnarls are suppressed, and fatty acids are not accumulated in the absence of peroxisomal membranes. Our results suggest that the extensive physical contact between peroxisomes and lipid bodies promotes the coupling of lipolysis within lipid bodies with peroxisomal fatty acid oxidation.
Figures






References
-
- Athenstaedt, K., and G. Daum. 2005. Tgl4p and Tgl5p, two triacylglycerol lipases of the yeast Saccharomyces cerevisiae are localized to lipid particles. J. Biol. Chem. 280:37301–37309. - PubMed
-
- Baerends, R.J., S.W. Rasmussen, R.E. Hilbrands, M. van der Heide, K.N. Faber, P.T. Reuvekamp, J.A. Kiel, J.M. Cregg, I.J. van der Klei, and M. Veenhuis. 1996. The Hansenula polymorpha PER9 gene encodes a peroxisomal membrane protein essential for peroxisome assembly and integrity. J. Biol. Chem. 271:8887–8894. - PubMed
-
- Battaile, K.P., J. Molin-Case, R. Paschke, M. Wang, D. Bennett, J. Vockley, and J.J. Kim. 2002. Crystal structure of rat short chain acyl-CoA dehydrogenase complexed with acetoacetyl-CoA: comparison with other acyl-CoA dehydrogenases. J. Biol. Chem. 277:12200–12207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

