Fetal hemoglobin silencing in humans
- PMID: 16735596
- PMCID: PMC1895549
- DOI: 10.1182/blood-2006-04-015859
Fetal hemoglobin silencing in humans
Abstract
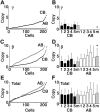
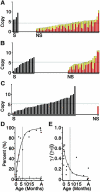
Interruption of the normal fetal-to-adult transition of hemoglobin expression should largely ameliorate sickle cell and beta-thalassemia syndromes. Achievement of this clinical goal requires a robust understanding of gamma-globin gene and protein silencing during human development. For this purpose, age-related changes in globin phenotypes of circulating human erythroid cells were examined from 5 umbilical cords, 99 infants, and 5 adult donors. Unexpectedly, an average of 95% of the cord blood erythrocytes and reticulocytes expressed HbA and the adult beta-globin gene, as well as HbF and the gamma-globin genes. The distribution of hemoglobin and globin gene expression then changed abruptly due to the expansion of cells lacking HbF or gamma-globin mRNA (silenced cells). In adult reticulocytes, less than 5% expressed gamma-globin mRNA. These data are consistent with a "switching" model in humans that initially results largely from gamma- and beta-globin gene coexpression and competition during fetal development. In contrast, early postnatal life is marked by the rapid accumulation of cells that possess undetectable gamma-globin mRNA and HbF. The silencing phenomenon is mediated by a mechanism of cellular replacement. This novel silencing pattern may be important for the development of HbF-enhancing therapies.
Figures
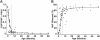


References
-
- Bard H, Fouron JC, Gagnon C, Gagnon J. Hypoxemia and increased fetal hemoglobin synthesis. J Pediatr. 1994;124: 941-943. - PubMed
-
- Perrine SP, Greene MF, Faller DV. Delay in the fetal globin switch in infants of diabetic mothers. N Engl J Med. 1985;312: 334-338. - PubMed
-
- Di Benedetto A, Romano G, Campo S, Di Cesare E, Cucinotta D. No evidence of increased fetal haemoglobin in an adult diabetic population. Diabetes Res Clin Pract. 1996;33: 37-41. - PubMed
-
- Watson J, Stahman A, Bilello F. The significance of the paucity of sickle cells in newborn negro infants. Am J Med Sci. 1948;215: 419-423. - PubMed
-
- Jacob GF, Raper AB. Hereditary persistence of foetal haemoglobin production, and its interaction with the sickle-cell trait. Br J Haematol. 1958;4: 138-149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

