Calmodulin contributes to gating control in olfactory calcium-activated chloride channels
- PMID: 16735757
- PMCID: PMC2151545
- DOI: 10.1085/jgp.200609497
Calmodulin contributes to gating control in olfactory calcium-activated chloride channels
Abstract
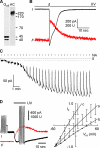
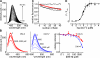
In sensory neurons of the peripheral nervous system, receptor potentials can be amplified by depolarizing Cl currents. In mammalian olfactory sensory neurons (OSNs), this anion-based signal amplification results from the sequential activation of two distinct types of transduction channels: cAMP-gated Ca channels and Ca-activated Cl channels. The Cl current increases the initial receptor current about 10-fold and leads to the excitation of the neuron. Here we examine the activation mechanism of the Ca-dependent Cl channel. We focus on calmodulin, which is known to mediate Ca effects on various ion channels. We show that the cell line Odora, which is derived from OSN precursor cells in the rat olfactory epithelium, expresses Ca-activated Cl channels. Single-channel conductance, ion selectivity, voltage dependence, sensitivity to niflumic acid, and Ca sensitivity match between Odora channels and OSN channels. Transfection of Odora cells with CaM mutants reduces the Ca sensitivity of the Cl channels. This result points to the participation of calmodulin in the gating process of Ca-ativated Cl channels, and helps to understand how signal amplification works in the olfactory sensory cilia. Calmodulin was previously shown to mediate feedback inhibition of cAMP-synthesis and of the cAMP-gated Ca channels in OSNs. Our results suggest that calmodulin may also be instrumental in the generation of the excitatory Cl current. It appears to play a pivotal role in the peripheral signal processing of olfactory sensory information. Moreover, recent results from other peripheral neurons, as well as from smooth muscle cells, indicate that the calmodulin-controlled, anion-based signal amplification operates in various cell types where it converts Ca signals into membrane depolarization.
Figures




Similar articles
-
Bestrophin-2 is a candidate calcium-activated chloride channel involved in olfactory transduction.Proc Natl Acad Sci U S A. 2006 Aug 22;103(34):12929-34. doi: 10.1073/pnas.0604505103. Epub 2006 Aug 15. Proc Natl Acad Sci U S A. 2006. PMID: 16912113 Free PMC article.
-
Calcium signalling and regulation in olfactory neurons.Curr Opin Neurobiol. 1999 Aug;9(4):419-26. doi: 10.1016/S0959-4388(99)80063-4. Curr Opin Neurobiol. 1999. PMID: 10448159 Review.
-
Temporal development of cyclic nucleotide-gated and Ca2+ -activated Cl- currents in isolated mouse olfactory sensory neurons.J Neurophysiol. 2007 Jul;98(1):153-60. doi: 10.1152/jn.00270.2007. Epub 2007 Apr 25. J Neurophysiol. 2007. PMID: 17460108
-
Single Ca(2+)-activated Cl(-) channel currents recorded from toad olfactory cilia.BMC Neurosci. 2016 Apr 25;17(1):17. doi: 10.1186/s12868-016-0252-0. BMC Neurosci. 2016. PMID: 27113933 Free PMC article.
-
Anoctamin 2/TMEM16B: a calcium-activated chloride channel in olfactory transduction.Exp Physiol. 2012 Feb;97(2):193-9. doi: 10.1113/expphysiol.2011.058230. Epub 2011 Sep 2. Exp Physiol. 2012. PMID: 21890523 Review.
Cited by
-
Direct or indirect regulation of calcium-activated chloride channel by calcium.J Membr Biol. 2011 Apr;240(3):121-9. doi: 10.1007/s00232-011-9350-1. Epub 2011 Mar 19. J Membr Biol. 2011. PMID: 21424226
-
Preassociated apocalmodulin mediates Ca2+-dependent sensitization of activation and inactivation of TMEM16A/16B Ca2+-gated Cl- channels.Proc Natl Acad Sci U S A. 2014 Dec 23;111(51):18213-8. doi: 10.1073/pnas.1420984111. Epub 2014 Dec 8. Proc Natl Acad Sci U S A. 2014. PMID: 25489088 Free PMC article.
-
Ca extrusion by NCX is compromised in olfactory sensory neurons of OMP mice.PLoS One. 2009;4(1):e4260. doi: 10.1371/journal.pone.0004260. Epub 2009 Jan 23. PLoS One. 2009. PMID: 19165324 Free PMC article.
-
Ca2+-activated Cl- channels at a glance.J Cell Sci. 2012 Mar 15;125(Pt 6):1367-71. doi: 10.1242/jcs.093260. J Cell Sci. 2012. PMID: 22526416 Free PMC article. Review. No abstract available.
-
Calmodulin regulation of TMEM16A and 16B Ca(2+)-activated chloride channels.Channels (Austin). 2016;10(1):38-44. doi: 10.1080/19336950.2015.1058455. Epub 2015 Jun 17. Channels (Austin). 2016. PMID: 26083059 Free PMC article. Review.
References
-
- Ault, B., and L.M. Hildebrand. 1994. GABAA receptor-mediated excitation of nociceptive afferents in the rat isolated spinal cord-tail preparation. Neuropharmacology. 33:109–114. - PubMed
-
- Alvarez-Leefmans, F.J., M. Leon-Olea, J. Mendoza-Sotelo, F.J. Alvarez, B. Anton, and R. Garduno. 2001. Immunolocalization of the Na+-K+-2Cl− cotransporter in peripheral nervous tissue of vertebrates. Neuroscience. 104:569–582. - PubMed

