Pharmacological characterization of endocannabinoid transport and fatty acid amide hydrolase inhibitors
- PMID: 16736384
- PMCID: PMC11881822
- DOI: 10.1007/s10571-006-9072-6
Pharmacological characterization of endocannabinoid transport and fatty acid amide hydrolase inhibitors
Abstract
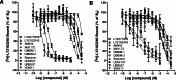
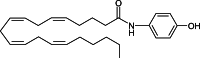
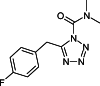
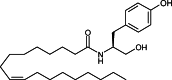
: 1. The mechanism of anandamide uptake and disposal has been an issue of considerable debate in the cannabinoid field. Several compounds have been reported to inhibit anandamide uptake or fatty acid amide hydrolase (FAAH; the primary catabolic enzyme of anandamide) activity with varying degrees of potency and selectivity. We recently reported the first evidence of a binding site involved in the uptake of endocannabinoids that is independent from FAAH. There are no direct comparisons of purported selective inhibitory compounds in common assay conditions measuring anandamide uptake, FAAH activity and binding activity. 2. A subset of compounds reported in the literature were tested in our laboratory under common assay conditions to measure their ability to (a) inhibit [(14)C]-anandamide uptake in cells containing (RBL-2H3) or cells lacking (HeLa) FAAH, (b) inhibit purified FAAH hydrolytic activity, and (c) inhibit binding to a putative binding site involved in endocannabinoid transport in both RBL and HeLa cell membranes. 3. Under these conditions, nearly all compounds tested inhibited (a) uptake of [(14)C]-anandamide, (b) enzyme activity in purified FAAH preparations, and (c) radioligand binding of [(3)H]-LY2183240 in RBL and HeLa plasma membrane preparations. General rank order potency was preserved within the three assays. However, concentration response curves were right-shifted for functional [(14)C]-anandamide uptake in HeLa (FAAH(-/-)) cells. 4. A more direct comparison of multiple inhibitors could be made in these three assay systems performed in the same laboratory, revealing more information about the selectivity of these compounds and the relationship between the putative endocannabinoid transport protein and FAAH. At least two separate proteins appear to be involved in uptake and degradation of anandamide. The most potent inhibitory compounds were right-shifted when transport was measured in HeLa (FAAH(-/-)) cells suggesting a requirement for a direct interaction with the FAAH protein to maintain high affinity binding of anandamide or inhibitors to the putative anandamide transport protein.
Figures







Similar articles
-
Role of fatty acid amide hydrolase in the transport of the endogenous cannabinoid anandamide.Mol Pharmacol. 2001 Jun;59(6):1369-75. doi: 10.1124/mol.59.6.1369. Mol Pharmacol. 2001. PMID: 11353795
-
Inhibition of fatty acid amide hydrolase and monoacylglycerol lipase by the anandamide uptake inhibitor VDM11: evidence that VDM11 acts as an FAAH substrate.Br J Pharmacol. 2005 Aug;145(7):885-93. doi: 10.1038/sj.bjp.0706253. Br J Pharmacol. 2005. PMID: 15895107 Free PMC article.
-
A role for caveolae/lipid rafts in the uptake and recycling of the endogenous cannabinoid anandamide.J Biol Chem. 2004 Oct 1;279(40):41991-7. doi: 10.1074/jbc.M407250200. Epub 2004 Aug 3. J Biol Chem. 2004. PMID: 15292270
-
The fatty acid amide hydrolase (FAAH).Prostaglandins Leukot Essent Fatty Acids. 2002 Feb-Mar;66(2-3):201-10. doi: 10.1054/plef.2001.0358. Prostaglandins Leukot Essent Fatty Acids. 2002. PMID: 12052036 Review.
-
Fatty acid amide hydrolase: an emerging therapeutic target in the endocannabinoid system.Curr Opin Chem Biol. 2003 Aug;7(4):469-75. doi: 10.1016/s1367-5931(03)00079-6. Curr Opin Chem Biol. 2003. PMID: 12941421 Review.
Cited by
-
Luciferin Amides Enable in Vivo Bioluminescence Detection of Endogenous Fatty Acid Amide Hydrolase Activity.J Am Chem Soc. 2015 Jul 15;137(27):8684-7. doi: 10.1021/jacs.5b04357. Epub 2015 Jul 2. J Am Chem Soc. 2015. PMID: 26120870 Free PMC article.
-
Pharmacotherapeutic modulation of the endocannabinoid signalling system in psychiatric disorders: drug-discovery strategies.Int Rev Psychiatry. 2009 Apr;21(2):122-33. doi: 10.1080/09540260902782778. Int Rev Psychiatry. 2009. PMID: 19367506 Free PMC article. Review.
-
Goods and Bads of the Endocannabinoid System as a Therapeutic Target: Lessons Learned after 30 Years.Pharmacol Rev. 2023 Sep;75(5):885-958. doi: 10.1124/pharmrev.122.000600. Epub 2023 May 10. Pharmacol Rev. 2023. PMID: 37164640 Free PMC article. Review.
-
Enzymatic pathways that regulate endocannabinoid signaling in the nervous system.Chem Rev. 2008 May;108(5):1687-707. doi: 10.1021/cr0782067. Epub 2008 Apr 23. Chem Rev. 2008. PMID: 18429637 Free PMC article. Review. No abstract available.
-
Inhibitors of Fatty Acid Amide Hydrolase and Monoacylglycerol Lipase: New Targets for Future Antidepressants.Curr Neuropharmacol. 2015;13(6):760-75. doi: 10.2174/1570159x13666150612225212. Curr Neuropharmacol. 2015. PMID: 26630956 Free PMC article. Review.
References
-
- Aquila, B. A., Hopkins, S., Lockshin, C. A., and Wang, F. (2003). Sepracor. Amines that inhibit a mammalian anandamide transporter, and methods of use thereof. WO03097573.
-
- Baker, D., Pryce, G., Davies, W. L., and Hiley, C. R. (2006). In silico patent searching reveals a new cannabinoid receptor. Trends Pharmacol. Sci.27(1):1–4. - PubMed
-
- Begg, M., Pacher, P., Batkai, S., Osei-Hyiaman, D., Offertaler, L., Mo, F. M., Liu, J., and Kunos, G. (2005). Evidence for novel cannabinoid receptors. Pharmacol. Ther.106(2):133–145. - PubMed
-
- Beltramo, M., Stella, N., Calignano, A., Lin, S. Y., Makriyannis, A., and Piomelli, D. (1997). Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science277(5329):1094–1097. - PubMed
-
- Boyd, S. T., and Fremming, B. A. (2005). Rimonabant–a selective CB1 antagonist. Ann. Pharmacother.39(4):684–690. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

