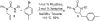A new synthesis of imidazolidin-2-ones via Pd-catalyzed carboamination of N-allylureas
- PMID: 16737306
- PMCID: PMC2527974
- DOI: 10.1021/ol060707b
A new synthesis of imidazolidin-2-ones via Pd-catalyzed carboamination of N-allylureas
Abstract
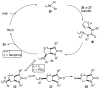
A new strategy for the preparation of substituted imidazolidin-2-ones in two steps from readily available N-allylamines is described. Addition of the amine starting materials to isocyanates affords N-allylureas, which are converted to imidazolidin-2-one products with generation of two bonds and up to two stereocenters when treated with aryl bromides and catalytic amounts of Pd2(dba)3/Xantphos in the presence of NaO(t)Bu. [reaction: see text]
References
-
- Kim J-M, Wilson TE, Norman TC, Schultz PG. Tetrahedron Lett. 1996;37:5309–5312.
-
- Kazmierski WM, Furfine E, Gray-Nunez Y, Spaltenstein A, Wright L. Bioorg Med Chem Lett. 2004;14:5685–5687. - PubMed
-
- DeLucca GV, Lam PYS. Drugs Fut. 1998;23:987–994.
-
- Sartori G, Maggi R. In: Science of Synthesis (Houben-Weyl Methods of Molecular Transformations) Ley SV, Knight JG, editors. Vol. 18. Thieme; Stuttgart: 2005. pp. 665–758.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources