A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients
- PMID: 16737553
- PMCID: PMC1557737
- DOI: 10.1186/bcr1412
A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients
Abstract
Introduction: The Oncotype DX assay was recently reported to predict risk for distant recurrence among a clinical trial population of tamoxifen-treated patients with lymph node-negative, estrogen receptor (ER)-positive breast cancer. To confirm and extend these findings, we evaluated the performance of this 21-gene assay among node-negative patients from a community hospital setting.
Methods: A case-control study was conducted among 4,964 Kaiser Permanente patients diagnosed with node-negative invasive breast cancer from 1985 to 1994 and not treated with adjuvant chemotherapy. Cases (n = 220) were patients who died from breast cancer. Controls (n = 570) were breast cancer patients who were individually matched to cases with respect to age, race, adjuvant tamoxifen, medical facility and diagnosis year, and were alive at the date of death of their matched case. Using an RT-PCR assay, archived tumor tissues were analyzed for expression levels of 16 cancer-related and five reference genes, and a summary risk score (the Recurrence Score) was calculated for each patient. Conditional logistic regression methods were used to estimate the association between risk of breast cancer death and Recurrence Score.
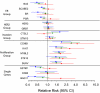
Results: After adjusting for tumor size and grade, the Recurrence Score was associated with risk of breast cancer death in ER-positive, tamoxifen-treated and -untreated patients (P = 0.003 and P = 0.03, respectively). At 10 years, the risks for breast cancer death in ER-positive, tamoxifen-treated patients were 2.8% (95% confidence interval [CI] 1.7-3.9%), 10.7% (95% CI 6.3-14.9%), and 15.5% (95% CI 7.6-22.8%) for those in the low, intermediate and high risk Recurrence Score groups, respectively. They were 6.2% (95% CI 4.5-7.9%), 17.8% (95% CI 11.8-23.3%), and 19.9% (95% CI 14.2-25.2%) for ER-positive patients not treated with tamoxifen. In both the tamoxifen-treated and -untreated groups, approximately 50% of patients had low risk Recurrence Score values.
Conclusion: In this large, population-based study of lymph node-negative patients not treated with chemotherapy, the Recurrence Score was strongly associated with risk of breast cancer death among ER-positive, tamoxifen-treated and -untreated patients.
Figures


References
-
- Ghafoor A, Jemal A, Ward E, Cokkinides V, Smith R, Thun M. Trends in breast cancer by race and ethnicity. CA Cancer J Clin. 2003;53:342–355. - PubMed
-
- Fisher B, Jeong JH, Bryant J, Anderson S, Dignam J, Fisher ER, Wolmark N. National Surgical Adjuvant Breast and Bowel Project randomised clinical trials: Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet. 2004;364:858–868. doi: 10.1016/S0140-6736(04)16981-X. - DOI - PubMed
-
- Eifel P, Axelson JA, Costa J, Crowley J, Curran WJ, Jr, Deshler A, Fulton S, Hendricks CB, Kemeny M, Kornblith AB, et al. National Institutes of Health Consensus Development Conference Statement: adjuvant therapy for breast cancer, November 1–3, 2000. J Natl Cancer Inst. 2001;93:979–989. doi: 10.1093/jnci/93.13.979. - DOI - PubMed
-
- Goldhirsch A, Glick JH, Gelber RD, Coates AS, Senn HJ. Meeting highlights: International Consensus Panel on the Treatment of Primary Breast Cancer. Seventh International Conference on Adjuvant Therapy of Primary Breast Cancer. J Clin Oncol. 2001;19:3817–3827. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

