Autosomal recessive retinitis pigmentosa and E150K mutation in the opsin gene
- PMID: 16737970
- PMCID: PMC1618956
- DOI: 10.1074/jbc.M602664200
Autosomal recessive retinitis pigmentosa and E150K mutation in the opsin gene
Abstract
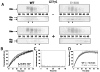
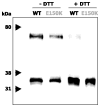
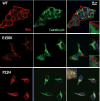
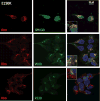
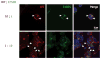
Retinitis pigmentosa (RP) is a heterogeneous group of hereditary disorders of the retina caused by mutation in genes of the photoreceptor proteins with an autosomal dominant (adRP), autosomal recessive (arRP), or X-linked pattern of inheritance. Although there are over 100 identified mutations in the opsin gene associated with RP, only a few of them are inherited with the arRP pattern. E150K is the first reported missense mutation associated with arRP. This opsin mutation is located in the second cytoplasmic loop of this G protein-coupled receptor. E150K opsin expressed in HEK293 cells and reconstituted with 11-cis-retinal displayed an absorption spectrum similar to the wild type (WT) counterpart and activated G protein transducin slightly faster than WT receptor. However, the majority of E150K opsin showed a higher apparent molecular mass in SDS-PAGE and was resistant to endoglycosidase H deglycosidase. Instead of being transported to the plasma membrane, E150K opsin is partially colocalized with the cis/medial Golgi compartment markers such as GM130 and Vti1b but not with the trans-Golgi network. In contrast to the endoplasmic reticulum-retained adRP mutant, P23H opsin, Golgi-retained E150K opsin did not influence the proper transport of the WT opsin when coexpressed in HEK293 cells. This result is consistent with the recessive pattern of inheritance of this mutation. Thus, our study reveals a novel molecular mechanism for retinal degeneration that results from deficient export of opsin from the Golgi apparatus.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

