Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging
- PMID: 16738054
- PMCID: PMC1472659
- DOI: 10.1073/pnas.0602569103
Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging
Abstract
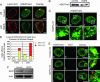
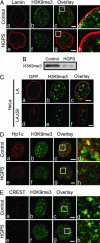
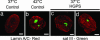
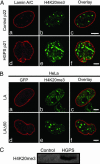
The premature aging disease Hutchinson-Gilford Progeria Syndrome (HGPS) is caused by a mutant lamin A (LADelta50). Nuclei in cells expressing LADelta50 are abnormally shaped and display a loss of heterochromatin. To determine the mechanisms responsible for the loss of heterochromatin, epigenetic marks regulating either facultative or constitutive heterochromatin were examined. In cells from a female HGPS patient, histone H3 trimethylated on lysine 27 (H3K27me3), a mark for facultative heterochromatin, is lost on the inactive X chromosome (Xi). The methyltransferase responsible for this mark, EZH2, is also down-regulated. These alterations are detectable before the changes in nuclear shape that are considered to be the pathological hallmarks of HGPS cells. The results also show a down-regulation of the pericentric constitutive heterochromatin mark, histone H3 trimethylated on lysine 9, and an altered association of this mark with heterochromatin protein 1alpha (Hp1alpha) and the CREST antigen. This loss of constitutive heterochromatin is accompanied by an up-regulation of pericentric satellite III repeat transcripts. In contrast to these decreases in histone H3 methylation states, there is an increase in the trimethylation of histone H4K20, an epigenetic mark for constitutive heterochromatin. Expression of LADelta50 in normal cells induces changes in histone methylation patterns similar to those seen in HGPS cells. The epigenetic changes described most likely represent molecular mechanisms responsible for the rapid progression of premature aging in HGPS patients.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

References
-
- Gilford M. Practitioner. 1904;73:188–217.
-
- de Sandre-Giovannoli A., Bernard R., Cau P., Navarro C., Amiel J., Boccaccio I., Lyonnet S., Stewart C. L., Munnich A., Le Merrer M., Levy N. Science. 2003;300:2055. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

