Neuromodulation of spike-timing precision in sensory neurons
- PMID: 16738233
- PMCID: PMC6675233
- DOI: 10.1523/JNEUROSCI.4659-05.2006
Neuromodulation of spike-timing precision in sensory neurons
Abstract
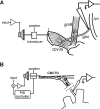
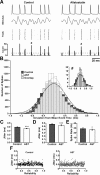
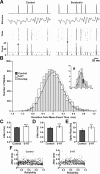
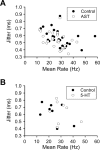
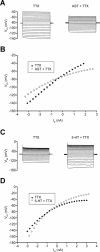
The neuropeptide allatostatin decreases the spike rate in response to time-varying stretches of two different crustacean mechanoreceptors, the gastropyloric receptor 2 in the crab Cancer borealis and the coxobasal chordotonal organ (CBCTO) in the crab Carcinus maenas. In each system, the decrease in firing rate is accompanied by an increase in the timing precision of spikes triggered by discrete temporal features in the stimulus. This was quantified by calculating the standard deviation or "jitter" in the times of individual identified spikes elicited in response to repeated presentations of the stimulus. Conversely, serotonin increases the firing rate but decreases the timing precision of the CBCTO response. Intracellular recordings from the afferents of this receptor demonstrate that allatostatin increases the conductance of the neurons, consistent with its inhibitory action on spike rate, whereas serotonin decreases the overall membrane conductance. We conclude that spike-timing precision of mechanoreceptor afferents in response to dynamic stimulation can be altered by neuromodulators acting directly on the afferent neurons.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
