Lymphocyte chemotaxis is regulated by histone deacetylase 6, independently of its deacetylase activity
- PMID: 16738306
- PMCID: PMC1525231
- DOI: 10.1091/mbc.e06-01-0008
Lymphocyte chemotaxis is regulated by histone deacetylase 6, independently of its deacetylase activity
Abstract
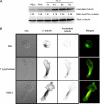
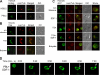
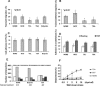
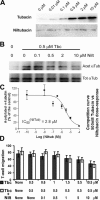
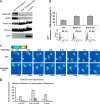
In this work, the role of HDAC6, a type II histone deacetylase with tubulin deacetylase activity, in lymphocyte polarity, motility, and transmigration was explored. HDAC6 was localized at dynamic subcellular structures as leading lamellipodia and the uropod in migrating T-cells. However, HDAC6 activity did not appear to be involved in the polarity of migrating lymphocytes. Overexpression of HDAC6 in freshly isolated lymphocytes and T-cell lines increased the lymphocyte migration mediated by chemokines and their transendothelial migration under shear flow. Accordingly, the knockdown of HDAC6 expression in T-cells diminished their chemotactic capability. Additional experiments with HDAC6 inhibitors (trichostatin, tubacin), other structural related molecules (niltubacin, MAZ-1391), and HDAC6 dead mutants showed that the deacetylase activity of HDAC6 was not involved in the modulatory effect of this molecule on cell migration. Our results indicate that HDAC6 has an important role in the chemotaxis of T-lymphocytes, which is independent of its tubulin deacetylase activity.
Figures

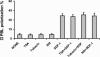
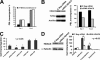
Similar articles
-
Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation.Proc Natl Acad Sci U S A. 2003 Apr 15;100(8):4389-94. doi: 10.1073/pnas.0430973100. Epub 2003 Apr 3. Proc Natl Acad Sci U S A. 2003. PMID: 12677000 Free PMC article.
-
The histone deacetylase-6 inhibitor tubacin directly inhibits de novo sphingolipid biosynthesis as an off-target effect.Biochem Biophys Res Commun. 2014 Jul 4;449(3):268-71. doi: 10.1016/j.bbrc.2014.05.016. Epub 2014 May 14. Biochem Biophys Res Commun. 2014. PMID: 24835950
-
Histone deacetylase 6 inhibits influenza A virus release by downregulating the trafficking of viral components to the plasma membrane via its substrate, acetylated microtubules.J Virol. 2014 Oct;88(19):11229-39. doi: 10.1128/JVI.00727-14. Epub 2014 Jul 16. J Virol. 2014. PMID: 25031336 Free PMC article.
-
Beyond the Selective Inhibition of Histone Deacetylase 6.Mini Rev Med Chem. 2016;16(14):1175-84. doi: 10.2174/1389557516666160428115959. Mini Rev Med Chem. 2016. PMID: 27121714 Review.
-
Histone deacetylase inhibitors that target tubulin.Cancer Lett. 2009 Aug 8;280(2):222-32. doi: 10.1016/j.canlet.2009.01.040. Epub 2009 Mar 5. Cancer Lett. 2009. PMID: 19268440 Review.
Cited by
-
Reactivation of DNA viruses in association with histone deacetylase inhibitor therapy: a case series report.Haematologica. 2009 Nov;94(11):1618-22. doi: 10.3324/haematol.2009.008607. Epub 2009 Jul 16. Haematologica. 2009. PMID: 19608677 Free PMC article. Clinical Trial.
-
Post-translational modification-regulated leukocyte adhesion and migration.Oncotarget. 2016 Jun 14;7(24):37347-37360. doi: 10.18632/oncotarget.8135. Oncotarget. 2016. PMID: 26993608 Free PMC article. Review.
-
Destabilization of ERBB2 transcripts by targeting 3' untranslated region messenger RNA associated HuR and histone deacetylase-6.Mol Cancer Res. 2008 Jul;6(7):1250-8. doi: 10.1158/1541-7786.MCR-07-2110. Mol Cancer Res. 2008. PMID: 18644987 Free PMC article.
-
Chasing a Breath of Fresh Air in Cystic Fibrosis (CF): Therapeutic Potential of Selective HDAC6 Inhibitors to Tackle Multiple Pathways in CF Pathophysiology.J Med Chem. 2022 Feb 24;65(4):3080-3097. doi: 10.1021/acs.jmedchem.1c02067. Epub 2022 Feb 11. J Med Chem. 2022. PMID: 35148101 Free PMC article. Review.
-
Identification of HDAC6-Selective Inhibitors of Low Cancer Cell Cytotoxicity.ChemMedChem. 2016 Jan 5;11(1):81-92. doi: 10.1002/cmdc.201500456. Epub 2015 Nov 23. ChemMedChem. 2016. PMID: 26592932 Free PMC article.
References
-
- Alon R., Feigelson S. From rolling to arrest on blood vessels: leukocyte tap dancing on endothelial integrin ligands and chemokines at sub-second contacts. Semin. Immunol. 2002;14:93–104. - PubMed
-
- Bali P., et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90, a novel basis for antileukemia activity of histone deacetylase inhibitors. J. Biol. Chem. 2005;280:26729–26734. - PubMed
-
- Barreiro O., Yanez-Mo M., Sala-Valdes M., Gutierrez-Lopez M. D., Ovalle S., Higginbottom A., Monk P. N., Cabanas C., Sanchez-Madrid F. Endothelial tetraspanin microdomains regulate leukocyte firm adhesion during extravasation. Blood. 2005;105:2852–2861. - PubMed
-
- Barreiro O., Yanez-Mo M., Serrador J. M., Montoya M. C., Vicente- Manzanares M., Tejedor R., Furthmayr H., Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J. Cell Biol. 2002;157:1233–1245. - PMC - PubMed
-
- Bertos N. R., Gilquin B., Chan G. K., Yen T. J., Khochbin S., Yang X. J. Role of the tetradecapeptide repeat domain of human histone deacetylase 6 in cytoplasmic retention. J. Biol. Chem. 2004;279:48246–48254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

