p18 Ink4c and Pten constrain a positive regulatory loop between cell growth and cell cycle control
- PMID: 16738322
- PMCID: PMC1489117
- DOI: 10.1128/MCB.00266-06
p18 Ink4c and Pten constrain a positive regulatory loop between cell growth and cell cycle control
Abstract
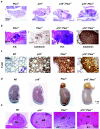
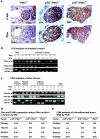
Inactivation of the Rb-mediated G1 control pathway is a common event found in many types of human tumors. To test how the Rb pathway interacts with other pathways in tumor suppression, we characterized mice with mutations in both the cyclin-dependent kinase (CDK) inhibitor p18 Ink4c and the lipid phosphatase Pten, which regulates cell growth. The double mutant mice develop a wider spectrum of tumors, including prostate cancer in the anterior and dorsolateral lobes, with nearly complete penetrance and at an accelerated rate. The remaining wild-type allele of Pten was lost at a high frequency in Pten+/- cells but not in p18+/- Pten+/- or p18-/- Pten+/- prostate tumor cells, nor in other Pten+/- tumor cells, suggesting a tissue- and genetic background-dependent haploinsufficiency of Pten in tumor suppression. p18 deletion, CDK4 overexpression, or oncoviral inactivation of Rb family proteins caused activation of Akt/PKB that was recessive to the reduction of PTEN activity. We suggest that p18 and Pten cooperate in tumor suppression by constraining a positive regulatory loop between cell growth and cell cycle control pathways.
Figures




References
-
- Abate-Shen, C., and M. M. Shen. 2000. Molecular genetics of prostate cancer. Genes Dev. 14:2410-2434. - PubMed
-
- Cantley, L. C. 2002. The phosphoinositide 3-kinase pathway. Science 296:1655-1657. - PubMed
-
- Chaussepied, M., and D. Ginsberg. 2004. Transcriptional regulation of AKT activation by E2F. Mol. Cell 16:831-837. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
