Slm1 and slm2 are novel substrates of the calcineurin phosphatase required for heat stress-induced endocytosis of the yeast uracil permease
- PMID: 16738335
- PMCID: PMC1489119
- DOI: 10.1128/MCB.01973-05
Slm1 and slm2 are novel substrates of the calcineurin phosphatase required for heat stress-induced endocytosis of the yeast uracil permease
Abstract
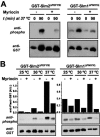
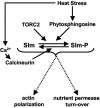
The Ca2+/calmodulin-dependent phosphatase calcineurin promotes yeast survival during environmental stress. We identified Slm1 and Slm2 as calcineurin substrates required for sphingolipid-dependent processes. Slm1 and Slm2 bind to calcineurin via docking sites that are required for their dephosphorylation by calcineurin and are related to the PXIXIT motif identified in NFAT. In vivo, calcineurin mediates prolonged dephosphorylation of Slm1 and Slm2 during heat stress, and this response can be mimicked by exogenous addition of the sphingoid base phytosphingosine. Slm proteins also promote the growth of yeast cells in the presence of myriocin, an inhibitor of sphingolipid biosynthesis, and regulation of Slm proteins by calcineurin is required for their full activity under these conditions. During heat stress, sphingolipids signal turnover of the uracil permease, Fur4. In cells lacking Slm protein activity, stress-induced endocytosis of Fur4 is blocked, and Fur4 accumulates at the cell surface in a ubiquitinated form. Furthermore, cells expressing a version of Slm2 that cannot be dephosphorylated by calcineurin display an increased rate of Fur4 turnover during heat stress. Thus, calcineurin may modulate sphingolipid-dependent events through regulation of Slm1 and Slm2. These findings, in combination with previous work identifying Slm1 and Slm2 as targets of Mss4/phosphatidylinositol 4,5-bisphosphate and TORC2 signaling, suggest that Slm proteins integrate information from a variety of signaling pathways to coordinate the cellular response to heat stress.
Figures






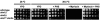


References
-
- Aramburu, J., F. Garcia-Cozar, A. Raghavan, H. Okamura, A. Rao, and P. G. Hogan. 1998. Selective inhibition of NFAT activation by a peptide spanning the calcineurin targeting site of NFAT. Mol. Cell 1:627-637. - PubMed
-
- Aramburu, J., A. Rao, and C. B. Klee. 2000. Calcineurin: from structure to function. Curr. Top. Cell. Regul. 36:237-295. - PubMed
-
- Aramburu, J., M. B. Yaffe, C. Lopez-Rodriguez, L. C. Cantley, P. G. Hogan, and A. Rao. 1999. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science 285:2129-2133. - PubMed
-
- Ausubel, F. M, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
