Repression of the antiapoptotic molecule galectin-3 by homeodomain-interacting protein kinase 2-activated p53 is required for p53-induced apoptosis
- PMID: 16738336
- PMCID: PMC1489111
- DOI: 10.1128/MCB.00959-05
Repression of the antiapoptotic molecule galectin-3 by homeodomain-interacting protein kinase 2-activated p53 is required for p53-induced apoptosis
Abstract
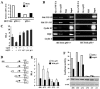
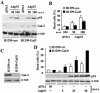
Galectin 3 (Gal-3), a member of the beta-galactoside binding lectin family, exhibits antiapoptotic functions, and its aberrant expression is involved in various aspects of tumor progression. Here we show that p53-induced apoptosis is associated with transcriptional repression of Gal-3. Previously, it has been reported that phosphorylation of p53 at Ser46 is important for transcription of proapoptotic genes and induction of apoptosis and that homeodomain-interacting protein kinase 2 (HIPK2) is specifically involved in these functions. We show that HIPK2 cooperates with p53 in Gal-3 repression and that this cooperation requires HIPK2 kinase activity. Gene-specific RNA interference demonstrates that HIPK2 is essential for repression of Gal-3 upon induction of p53-dependent apoptosis. Furthermore, expression of a nonrepressible Gal-3 prevents HIPK2- and p53-induced apoptosis. These results reveal a new apoptotic pathway induced by HIPK2-activated p53 and requiring repression of the antiapoptotic factor Gal-3.
Figures




References
-
- Akahani, S., P. Nangia-Makker, H. Inohara, H.-R. Kim, and A. Raz. 1997. Galectin-3: a novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res. 57:5272-5276. - PubMed
-
- Appella, E., and C. W. Anderson. 2000. Signaling to p53: breaking the post-translational modification code. Pathol. Biol. 48:227-245. - PubMed
-
- Bacchetti, S., and F. Graham. 1993. Inhibition of cell proliferation by an adenovirus vector expressing the human wild type p53 protein. Int. J. Oncol. 3:781-788. - PubMed
-
- Barondes, S. H., V. Castronovo, D. N. Cooper, R. D. Cummings, K. Drickamer, T. Feizi, M. A. Gitt, J. Hirabayashi, C. Hughes, K. Kasai, et al. 1994. Galectins: a family of animal beta-galactoside-binding lectins. Cell 76:597-598. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
