The proteomes of neurotransmitter receptor complexes form modular networks with distributed functionality underlying plasticity and behaviour
- PMID: 16738568
- PMCID: PMC1681474
- DOI: 10.1038/msb4100041
The proteomes of neurotransmitter receptor complexes form modular networks with distributed functionality underlying plasticity and behaviour
Abstract
Neuronal synapses play fundamental roles in information processing, behaviour and disease. Neurotransmitter receptor complexes, such as the mammalian N-methyl-D-aspartate receptor complex (NRC/MASC) comprising 186 proteins, are major components of the synapse proteome. Here we investigate the organisation and function of NRC/MASC using a systems biology approach. Systematic annotation showed that the complex contained proteins implicated in a wide range of cognitive processes, synaptic plasticity and psychiatric diseases. Protein domains were evolutionarily conserved from yeast, but enriched with signalling domains associated with the emergence of multicellularity. Mapping of protein-protein interactions to create a network representation of the complex revealed that simple principles underlie the functional organisation of both proteins and their clusters, with modularity reflecting functional specialisation. The known functional roles of NRC/MASC proteins suggest the complex co-ordinates signalling to diverse effector pathways underlying neuronal plasticity. Importantly, using quantitative data from synaptic plasticity experiments, our model correctly predicts robustness to mutations and drug interference. These studies of synapse proteome organisation suggest that molecular networks with simple design principles underpin synaptic signalling properties with important roles in physiology, behaviour and disease.
Figures
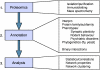
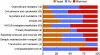

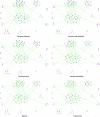


References
-
- Albert R, Jeong H, Barabasi AL (2000) Error and attack tolerance of complex networks. Nature 406: 378–382 - PubMed
-
- Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39 - PubMed
-
- Collins MO, Husi H, Yu L, Brandon JM, Anderson CNG, Blackstock WP, Choudhary J, Grant SGN (2005) Molecular characterization and comparison of the components and multi-protein complexes in the postsynaptic density. J Neurochemistry. Online Early, doi:10.1111/j.1471-4159.2005.03507.x - PubMed
-
- Csete M, Doyle J (2004) Bow ties, metabolism and disease. Trends Biotechnol 22: 446–450 - PubMed
-
- Enright AJ, Ouzounis CA (2001) BioLayout—an automatic graph layout algorithm for similarity visualization. Bioinformatics 17: 853–854 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

