Synthetic biology: new engineering rules for an emerging discipline
- PMID: 16738572
- PMCID: PMC1681505
- DOI: 10.1038/msb4100073
Synthetic biology: new engineering rules for an emerging discipline
Abstract
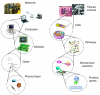
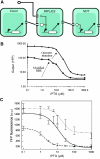
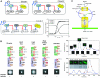
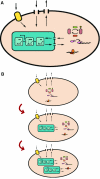
Synthetic biologists engineer complex artificial biological systems to investigate natural biological phenomena and for a variety of applications. We outline the basic features of synthetic biology as a new engineering discipline, covering examples from the latest literature and reflecting on the features that make it unique among all other existing engineering fields. We discuss methods for designing and constructing engineered cells with novel functions in a framework of an abstract hierarchy of biological devices, modules, cells, and multicellular systems. The classical engineering strategies of standardization, decoupling, and abstraction will have to be extended to take into account the inherent characteristics of biological devices and modules. To achieve predictability and reliability, strategies for engineering biology must include the notion of cellular context in the functional definition of devices and modules, use rational redesign and directed evolution for system optimization, and focus on accomplishing tasks using cell populations rather than individual cells. The discussion brings to light issues at the heart of designing complex living systems and provides a trajectory for future development.
Figures

References
-
- Alon U (2003) Biological networks: the tinkerer as an engineer. Science 301: 1866–1867 - PubMed
-
- Anderson JC, Clarke EJ, Arkin AP, Voigt CA (2005) Environmentally controlled invasion of cancer cells by engineered bacteria. J Mol Biol 355: 619–627 - PubMed
-
- Atkinson MR, Savageau MA, Myers JT, Ninfa AJ (2003) Development of genetic circuitry exhibiting toggle switch or oscillatory behavior in Escherichia coli. Cell 113: 597–607 - PubMed
-
- Balagadde FK, You L, Hansen CL, Arnold FH, Quake SR (2005) Long-term monitoring of bacteria undergoing programmed population control in a microchemostat. Science 309: 137–140 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

