Susceptibility to ozone-induced airway inflammation is associated with decreased levels of surfactant protein D
- PMID: 16740162
- PMCID: PMC1488844
- DOI: 10.1186/1465-9921-7-85
Susceptibility to ozone-induced airway inflammation is associated with decreased levels of surfactant protein D
Abstract
Background: Ozone (O3), a common air pollutant, induces exacerbation of asthma and chronic obstructive pulmonary disease. Pulmonary surfactant protein (SP)-D modulates immune and inflammatory responses in the lung. We have shown previously that SP-D plays a protective role in a mouse model of allergic airway inflammation. Here we studied the role and regulation of SP-D in O3-induced inflammatory changes in the lung.
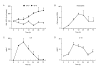
Methods: To evaluate the effects of O3 exposure in mouse strains with genetically different expression levels of SP-D we exposed Balb/c, C57BL/6 and SP-D knockout mice to O3 or air. BAL cellular and cytokine content and SP-D levels were evaluated and compared between the different strains. The kinetics of SP-D production and inflammatory parameters were studied at 0, 2, 6, 12, 24, 48, and 72 hrs after O3 exposure. The effect of IL-6, an O3-inducible cytokine, on the expression of SP-D was investigated in vitro using a primary alveolar type II cell culture.
Results: Ozone-exposed Balb/c mice demonstrated significantly enhanced acute inflammatory changes including recruitment of inflammatory cells and release of KC and IL-12p70 when compared with age- and sex-matched C57BL/6 mice. On the other hand, C57BL/6 mice had significantly higher levels of SP-D and released more IL-10 and IL-6. Increase in SP-D production coincided with the resolution of inflammatory changes. Mice deficient in SP-D had significantly higher numbers of inflammatory cells when compared to controls supporting the notion that SP-D has an anti-inflammatory function in our model of O3 exposure. IL-6, which was highly up-regulated in O3 exposed mice, was capable of inducing the expression of SP-D in vitro in a dose dependent manner.
Conclusion: Our data suggest that IL-6 contributes to the up-regulation of SP-D after acute O3 exposure and elevation of SP-D in the lung is associated with the resolution of inflammation. Absence or low levels of SP-D predispose to enhanced inflammatory changes following acute oxidative stress.
Figures
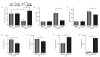
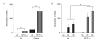
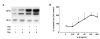
References
-
- Desqueyroux H, Pujet JC, Prosper M, Le Moullec Y, Momas I. Effects of air pollution on adults with chronic obstructive pulmonary disease. Arch Environ Health. 2002;57(6):554–560. - PubMed
-
- McKinney WJ, Jaskot RH, Richards JH, Costa DL, Dreher KL. Cytokine mediation of ozone-induced pulmonary adaptation. Am J Respir Cell Mol Biol. 1998;18(5):696–705. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

