RcsF is an outer membrane lipoprotein involved in the RcsCDB phosphorelay signaling pathway in Escherichia coli
- PMID: 16740933
- PMCID: PMC1482940
- DOI: 10.1128/JB.00004-06
RcsF is an outer membrane lipoprotein involved in the RcsCDB phosphorelay signaling pathway in Escherichia coli
Abstract
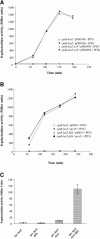
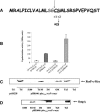
The RcsCDB signal transduction system is an atypical His-Asp phosphorelay conserved in gamma-proteobacteria. Besides the three proteins directly involved in the phosphorelay, two proteins modulate the activity of the system. One is RcsA, which can stimulate the activity of the response regulator RcsB independently of the phosphorelay to regulate a subset of RcsB targets. The other is RcsF, a putative outer membrane lipoprotein mediating the signaling to the sensor RcsC. How RcsF transduces the signal to RcsC is unknown. Although the molecular and physiological signals remain to be identified, the common feature among the reported Rcs-activating conditions is perturbation of the envelope. As an initial step to explore the RcsF-RcsC functional relationship, we demonstrate that RcsF is an outer membrane lipoprotein oriented towards the periplasm. We also report that a null mutation in surA, a gene required for correct folding of periplasmic proteins, activates the Rcs pathway through RcsF. In contrast, activation of this pathway by overproduction of the membrane chaperone-like protein DjlA does not require RcsF. Conversely, activation of the pathway by RcsF overproduction does not require DjlA either, indicating the existence of two independent signaling pathways toward RcsC.
Figures



References
-
- Behrens, S. 2002. Periplasmic chaperones-new structural and functional insights. Structure (Cambridge) 10:1469-1471. - PubMed
-
- Bernard, S., D. J. Clarke, M. X. Chen, I. B. Holland, and A. Jacq. 1998. Increased sensitivity of E. coli to novobiocin, EDTA and the anticalmodulin drug W7 following overproduction of DjlA requires a functional transmembrane domain. Mol. Gen. Genet. 259:645-655. - PubMed
-
- Clarke, D. J., I. B. Holland, and A. Jacq. 1997. Point mutations in the transmembrane domain of DjlA, a membrane-linked DnaJ-like protein, abolish its function in promoting colanic acid production via the Rcs signal transduction pathway. Mol. Microbiol. 25:933-944. - PubMed
-
- Clarke, D. J., A. Jacq, and I. B. Holland. 1996. A novel DnaJ-like protein in Escherichia coli inserts into the cytoplasmic membrane with a type III topology. Mol. Microbiol. 20:1273-1286. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

