Neutrophil cytoskeletal rearrangements during capillary sequestration in bacterial pneumonia in rats
- PMID: 16741152
- PMCID: PMC2648058
- DOI: 10.1164/rccm.200502-276OC
Neutrophil cytoskeletal rearrangements during capillary sequestration in bacterial pneumonia in rats
Abstract
Rationale: Neutrophils accumulate in pulmonary capillaries during acute inflammation. Initial events in injury recognition and sequestration do not occur through selectin-mediated rolling. Cytoskeletal rearrangements, as assessed by submembrane F-actin rims, result in poorly deformable neutrophils that may not pass through capillaries.
Objective: To test the hypothesis that neutrophils sequestering during pneumonia contain F-actin rims and to determine the roles of CD11/CD18, L-selectin expression, and neutrophil-platelet adhesion in neutrophil sequestration.
Methods: Neutrophils were compared in blood obtained simultaneously from venous and arterial sites before and 4 h after instillation of Streptococcus pneumoniae or Escherichia coli in rats.
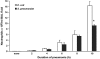
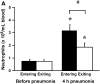
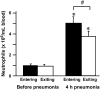
Measurements and main results: At 4 h of pneumonia, the number of neutrophils was greater in the venous blood entering the lungs than in the arterial blood leaving the lungs, indicating that neutrophil sequestration was occurring. More neutrophils entering the lungs contained F-actin rims than did neutrophils exiting, and the venous-arterial difference in F-actin-rimmed neutrophil counts completely accounted for sequestration. In E. coli pneumonia, in which neutrophil adhesion is mediated by CD11/CD18, CD18 blockade 15 min before blood samples were obtained did not prevent this sequestration of F-actin-rimmed neutrophils. Neutrophils expressing high or low levels of L-selectin or of neutrophils that bound platelets while circulating did not preferentially sequester.
Conclusions: Neutrophils with cytoskeletal rearrangements preferentially sequester within the lungs during pneumonia, and this sequestration is not due to CD11/CD18-mediated adhesion, L-selectin expression, or platelet adhesion to neutrophils, suggesting that cytoskeletal rearrangements result in sequestration of neutrophils.
Figures
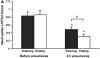
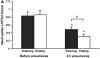
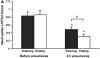
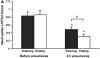





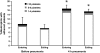
References
-
- Walker DC, Behzad AR, Chu F. Neutrophil migration through preexisting holes in the basal laminae of alveolar capillaries and epithelium during streptococcal pneumonia. Microvasc Res 1995;50:397–416. - PubMed
-
- Behzad AR, Chu F, Walker DC. Fibroblasts are in a position to provide directional information to migrating neutrophils during pneumonia in rabbit lungs. Microvasc Res 1996;51:303–316. - PubMed
-
- Downey GP, Worthen GS, Henson PM, Hyde DM. Neutrophil sequestration and migration in localized pulmonary inflammation: capillary localization and migration across the interalveolar septum. Am Rev Respir Dis 1993;147:168–176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

