Age-related enhancement of a protein synthesis-dependent late phase of LTP induced by low frequency paired-pulse stimulation in hippocampus
- PMID: 16741282
- PMCID: PMC1475810
- DOI: 10.1101/lm.166906
Age-related enhancement of a protein synthesis-dependent late phase of LTP induced by low frequency paired-pulse stimulation in hippocampus
Abstract
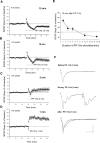
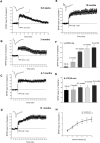
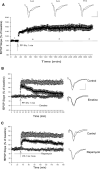
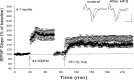
Protein synthesis-dependent late phase of LTP (L-LTP) is typically induced by repeated high-frequency stimulation (HFS). This form of L-LTP is reduced in the aged animal and is positively correlated with age-related memory loss. Here we report a novel form of protein synthesis-dependent late phase of LTP in the CA1 region of hippocampus induced by a brief 1-Hz paired-pulse stimulation (PP-1 Hz, 1 min). In contrast to L-LTP induced by HFS, the late phase of PP-1 Hz LTP does not exist in young adult animals. Rather, it emerges and becomes enhanced in an age-related way. Thus, in 1.5- to 2-mo-old mice, a brief PP-1 Hz stimulation induces only a short lasting LTP, decaying to baseline in about 90 min. By contrast, PP-1 Hz stimulation induces an enduring and protein synthesis dependent LTP in 12- to 18-mo-old mice. The PP-1 Hz-induced L-LTP is dependent on NMDA receptor activation, requires voltage-dependent calcium channels, and is modulated by dopamine D1/D5 receptors. Because memory ability declines with aging, the age-related enhancement of L-LTP induced by PP-1 Hz stimulation indicates that this form of L-LTP appears to be inversely correlated with memory ability.
Figures


Similar articles
-
Steroid pregnenolone sulfate enhances NMDA-receptor-independent long-term potentiation at hippocampal CA1 synapses: role for L-type calcium channels and sigma-receptors.Hippocampus. 2007;17(5):349-69. doi: 10.1002/hipo.20273. Hippocampus. 2007. PMID: 17330865
-
A form of long-lasting, learning-related synaptic plasticity in the hippocampus induced by heterosynaptic low-frequency pairing.Proc Natl Acad Sci U S A. 2004 Jan 20;101(3):859-64. doi: 10.1073/pnas.2237201100. Epub 2004 Jan 7. Proc Natl Acad Sci U S A. 2004. PMID: 14711997 Free PMC article.
-
Aging differentially alters forms of long-term potentiation in rat hippocampal area CA1.J Neurophysiol. 1998 Jan;79(1):334-41. doi: 10.1152/jn.1998.79.1.334. J Neurophysiol. 1998. PMID: 9425202
-
Synaptic strength at the temporoammonic input to the hippocampal CA1 region in vivo is regulated by NMDA receptors, metabotropic glutamate receptors and voltage-gated calcium channels.Neuroscience. 2015 Nov 19;309:191-9. doi: 10.1016/j.neuroscience.2015.03.014. Epub 2015 Mar 17. Neuroscience. 2015. PMID: 25791230
-
Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors.Crit Rev Neurobiol. 2006;18(1-2):71-84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. Crit Rev Neurobiol. 2006. PMID: 17725510 Review.
Cited by
-
Non-coding RNA transcripts: sensors of neuronal stress, modulators of synaptic plasticity, and agents of change in the onset of Alzheimer's disease.Neurosci Lett. 2009 Dec 4;466(2):81-8. doi: 10.1016/j.neulet.2009.08.032. Epub 2009 Aug 20. Neurosci Lett. 2009. PMID: 19699259 Free PMC article. Review.
-
Novel Players in the Aging Synapse: Impact on Cognition.J Caffeine Adenosine Res. 2019 Sep 1;9(3):104-127. doi: 10.1089/caff.2019.0013. Epub 2019 Sep 17. J Caffeine Adenosine Res. 2019. PMID: 31559391 Free PMC article. Review.
-
Impact of aging brain circuits on cognition.Eur J Neurosci. 2013 Jun;37(12):1903-15. doi: 10.1111/ejn.12183. Eur J Neurosci. 2013. PMID: 23773059 Free PMC article. Review.
-
Estradiol reverses a calcium-related biomarker of brain aging in female rats.J Neurosci. 2009 May 13;29(19):6058-67. doi: 10.1523/JNEUROSCI.5253-08.2009. J Neurosci. 2009. PMID: 19439583 Free PMC article.
-
Neural Protein Synthesis during Aging: Effects on Plasticity and Memory.Front Aging Neurosci. 2010 Aug 6;2:26. doi: 10.3389/fnagi.2010.00026. eCollection 2010. Front Aging Neurosci. 2010. PMID: 20802800 Free PMC article.
References
-
- Abel T., Nguyen P.V., Barad M., Deuel T.A., Kandel E.R., Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus based long-term memory. Cell. 1997;88:615–626. - PubMed
-
- Amenta F., Mignini F., Ricci A., Sabbatini M., Tomassoni D., Tayebati S.K. Age-related changes of dopamine receptors in the rat hippocampus: A light microscope autoradiography study. Mech. Ageing Dev. 2001;122:2071–2083. - PubMed
-
- Artola A., Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long term potentiation. Trends Neurosci. 1993;16:480–487. - PubMed
-
- Bach M.E., Barad M., Son H., Zhuo M., Lu Y.-F., Shih R., Mansuy I., Hawkins R.D., Kandel E.R. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and attenuated by drugs that enhance the cAMP signaling pathway. Proc. Natl. Acad. Sci. 1999;96:5280–5285. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
