Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions
- PMID: 16741504
- PMCID: PMC1479595
- DOI: 10.1038/sj.embor.7400705
Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions
Abstract
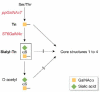
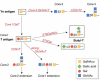
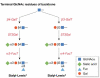
The glycoproteins of tumour cells are often abnormal, both in structure and in quantity. In particular, the mucin-type O-glycans have several cancer-associated structures, including the T and Tn antigens, and certain Lewis antigens. These structural changes can alter the function of the cell, and its antigenic and adhesive properties, as well as its potential to invade and metastasize. Cancer-associated mucin antigens can be exploited in diagnosis and prognosis, and in the development of cancer vaccines. The activities and Golgi localization of glycosyltransferases are the basis for the glycodynamics of cancer cells, and determine the ranges and amounts of specific O-glycans produced. This review focuses on the glycosyltransferases of colon and breast cancer cells that determine the pathways of mucin-type O-glycosylation, and the proposed functional and pathological consequences of altered O-glycans.
Figures


References
-
- Berois N, Mazal D, Ubillos L, Trajtenberg F, Nicolas A, Sastre-Garau X, Magdalenat H, Osinaga E (2006) UDP-N-acetylgalactosamine: polypeptide N-acetylgalactosaminyltransferase-6 as a new immunohistochemical breast cancer marker. J Histochem Cytochem 54: 317–328 - PubMed
-
- Blottiere HM, Burg C, Zennadi R, Perrin P, Blanchardie P, Bara J, Meflah K, LePendu J (1992) Involvement of histo-blood-group antigens in the susceptibility of colon carcinoma cells to natural killer-mediated cytotoxicity. Int J Cancer 52: 609–618 - PubMed
-
- Bresalier R, Ho S, Schoeppner H, Kim Y, Sleisenger M, Brodt P, Byrd J (1996) Enhanced sialyation of mucin-associated carbohydrate structures in human colon cancer metastasis. Gastroenterology 110: 1354–1367 - PubMed
-
- Brockhausen I (1999) Pathways of O-glycan biosynthesis in cancer cells. Biochim Biophys Acta 1473: 67–95 - PubMed
-
- Brockhausen I (2003a) Sulfotransferases involved in glycoprotein synthesis. Biochem Soc Trans 31: 318–325 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

