Integrating nuclear receptor mobility in models of gene regulation
- PMID: 16741568
- PMCID: PMC1472671
- DOI: 10.1621/nrs.04010
Integrating nuclear receptor mobility in models of gene regulation
Abstract
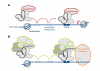
The mode of action of nuclear receptors in living cells is an actively investigated field but much remains hypothetical due to the lack, until recently, of methods allowing the assessment of molecular mechanisms in vivo. However, these last years, the development of fluorescence microscopy methods has allowed initiating the dissection of the molecular mechanisms underlying gene regulation by nuclear receptors directly in living cells or organisms. Following our analyses on peroxisome proliferator activated receptors (PPARs) in living cells, we discuss here the different models arising from the use of these tools, that attempt to link mobility, DNA binding or chromatin interaction, and transcriptional activity.
Figures

Similar articles
-
Transcription coactivators for peroxisome proliferator-activated receptors.Biochim Biophys Acta. 2007 Aug;1771(8):936-51. doi: 10.1016/j.bbalip.2007.01.008. Epub 2007 Jan 20. Biochim Biophys Acta. 2007. PMID: 17306620 Review.
-
Human mannose-binding lectin 2 is directly regulated by peroxisome proliferator-activated receptors via a peroxisome proliferator responsive element.J Biochem. 2013 Sep;154(3):265-73. doi: 10.1093/jb/mvt050. Epub 2013 May 27. J Biochem. 2013. PMID: 23711995
-
Interleukin-6 inhibits human peroxisome proliferator activated receptor alpha gene expression via CCAAT/enhancer-binding proteins in hepatocytes.Int J Biochem Cell Biol. 2007;39(10):1975-86. doi: 10.1016/j.biocel.2007.05.015. Epub 2007 May 31. Int J Biochem Cell Biol. 2007. PMID: 17616429
-
From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions.Prog Lipid Res. 2006 Mar;45(2):120-59. doi: 10.1016/j.plipres.2005.12.002. Epub 2006 Jan 25. Prog Lipid Res. 2006. PMID: 16476485 Review.
-
The nuclear receptor-coactivator interaction surface as a target for peptide antagonists of the peroxisome proliferator-activated receptors.Mol Endocrinol. 2007 Oct;21(10):2361-77. doi: 10.1210/me.2007-0201. Epub 2007 Jun 26. Mol Endocrinol. 2007. PMID: 17595321
Cited by
-
Increased peroxisome proliferator-activated receptor γ activity reduces imatinib uptake and efficacy in chronic myeloid leukemia mononuclear cells.Haematologica. 2017 May;102(5):843-853. doi: 10.3324/haematol.2016.153270. Epub 2017 Feb 2. Haematologica. 2017. PMID: 28154092 Free PMC article.
-
Live-cell fluorescence correlation spectroscopy dissects the role of coregulator exchange and chromatin binding in retinoic acid receptor mobility.J Cell Sci. 2011 Nov 1;124(Pt 21):3631-42. doi: 10.1242/jcs.086082. Epub 2011 Nov 1. J Cell Sci. 2011. PMID: 22045737 Free PMC article.
-
Ligand binding shifts highly mobile retinoid X receptor to the chromatin-bound state in a coactivator-dependent manner, as revealed by single-cell imaging.Mol Cell Biol. 2014 Apr;34(7):1234-45. doi: 10.1128/MCB.01097-13. Epub 2014 Jan 21. Mol Cell Biol. 2014. PMID: 24449763 Free PMC article.
-
Agonist-controlled competition of RAR and VDR nuclear receptors for heterodimerization with RXR is manifested in their DNA binding.J Biol Chem. 2023 Feb;299(2):102896. doi: 10.1016/j.jbc.2023.102896. Epub 2023 Jan 11. J Biol Chem. 2023. PMID: 36639026 Free PMC article.
References
-
- Agresti A., Scaffidi P., Riva A., Caiolfa V. R., Bianchi M. E. GR and HMGB1 interact only within chromatin and influence each other's residence time. Mol Cell. 2005;18:109–21. - PubMed
-
- Burakov D., Crofts L. A., Chang C. P., Freedman L. P. Reciprocal recruitment of DRIP/mediator and p160 coactivator complexes in vivo by estrogen receptor. J Biol Chem. 2002;277:14359–62. - PubMed
LinkOut - more resources
Full Text Sources

