Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction
- PMID: 16741575
- PMCID: PMC1464895
- DOI: 10.1172/JCI25397
Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction
Abstract
For over a century, there has been intense debate as to the reason why some cardiac stresses are pathological and others are physiological. One long-standing theory is that physiological overloads such as exercise are intermittent, while pathological overloads such as hypertension are chronic. In this study, we hypothesized that the nature of the stress on the heart, rather than its duration, is the key determinant of the maladaptive phenotype. To test this, we applied intermittent pressure overload on the hearts of mice and tested the roles of duration and nature of the stress on the development of cardiac failure. Despite a mild hypertrophic response, preserved systolic function, and a favorable fetal gene expression profile, hearts exposed to intermittent pressure overload displayed pathological features. Importantly, intermittent pressure overload caused diastolic dysfunction, altered beta-adrenergic receptor (betaAR) function, and vascular rarefaction before the development of cardiac hypertrophy, which were largely normalized by preventing the recruitment of PI3K by betaAR kinase 1 to ligand-activated receptors. Thus stress-induced activation of pathogenic signaling pathways, not the duration of stress or the hypertrophic growth per se, is the molecular trigger of cardiac dysfunction.
Figures

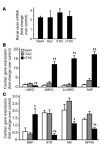

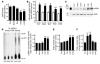




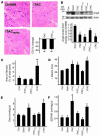

Comment in
-
Cardiac hypertrophy: stressing out the heart.J Clin Invest. 2006 Jun;116(6):1467-70. doi: 10.1172/JCI28884. J Clin Invest. 2006. PMID: 16741569 Free PMC article.
Similar articles
-
Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress.Circulation. 2002 Jan 1;105(1):85-92. doi: 10.1161/hc0102.101365. Circulation. 2002. PMID: 11772881
-
Physiological induction of a beta-adrenergic receptor kinase inhibitor transgene preserves ss-adrenergic responsiveness in pressure-overload cardiac hypertrophy.Circulation. 2000 Nov 28;102(22):2751-7. doi: 10.1161/01.cir.102.22.2751. Circulation. 2000. PMID: 11094043
-
Intermittent cardiac overload results in adaptive hypertrophy and provides protection against left ventricular acute pressure overload insult.J Physiol. 2015 Sep 1;593(17):3885-97. doi: 10.1113/JP270685. Epub 2015 Jun 23. J Physiol. 2015. PMID: 26010517 Free PMC article.
-
Induction of S100b in myocardium: an intrinsic inhibitor of cardiac hypertrophy.Can J Appl Physiol. 1998 Aug;23(4):377-89. doi: 10.1139/h98-022. Can J Appl Physiol. 1998. PMID: 9677434 Review.
-
The cardiac structure-function relationship and the renin-angiotensin-aldosterone system in hypertension and heart failure.Curr Opin Cardiol. 1994 Jul;9 Suppl 1:S2-10; discussion S10-1. doi: 10.1097/00001573-199407000-00002. Curr Opin Cardiol. 1994. PMID: 7827369 Review.
Cited by
-
Transverse aortic constriction induces gut barrier alterations, microbiota remodeling and systemic inflammation.Sci Rep. 2021 Apr 1;11(1):7404. doi: 10.1038/s41598-021-86651-y. Sci Rep. 2021. PMID: 33795775 Free PMC article.
-
Hypertrophic Preconditioning Attenuates Myocardial Ischaemia-Reperfusion Injury by Modulating SIRT3-SOD2-mROS-Dependent Autophagy.Cell Prolif. 2021 Jul;54(7):e13051. doi: 10.1111/cpr.13051. Epub 2021 May 11. Cell Prolif. 2021. PMID: 33973685 Free PMC article.
-
β-Arrestin-biased AT1R stimulation promotes cell survival during acute cardiac injury.Am J Physiol Heart Circ Physiol. 2012 Oct 15;303(8):H1001-10. doi: 10.1152/ajpheart.00475.2012. Epub 2012 Aug 10. Am J Physiol Heart Circ Physiol. 2012. PMID: 22886417 Free PMC article.
-
Anchored p90 ribosomal S6 kinase 3 is required for cardiac myocyte hypertrophy.Circ Res. 2013 Jan 4;112(1):128-39. doi: 10.1161/CIRCRESAHA.112.276162. Epub 2012 Sep 20. Circ Res. 2013. PMID: 22997248 Free PMC article.
-
Treatment of myocardial interstitial fibrosis in pathological myocardial hypertrophy.Front Pharmacol. 2022 Sep 30;13:1004181. doi: 10.3389/fphar.2022.1004181. eCollection 2022. Front Pharmacol. 2022. PMID: 36249793 Free PMC article. Review.
References
-
- Meerson F.Z. Compensatory hyperfunction of the heart and cardiac insufficiency. Circ. Res. 1962;10:250–258. - PubMed
-
- Levy D., Garrison R.J., Savage D.D., Kannel W.B., Castelli W.P. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N. Engl. J. Med. 1990;322:1561–1566. - PubMed
-
- Frey N., Katus H.A., Olson E.N., Hill J.A. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109:1580–1589. - PubMed
-
- Esposito G., et al. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation. 2002;105:85–92. - PubMed
-
- Yusuf S., et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N. Engl. J. Med. 2000;342:145–153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

