Combined ectopic expression of Pdx1 and Ptf1a/p48 results in the stable conversion of posterior endoderm into endocrine and exocrine pancreatic tissue
- PMID: 16751182
- PMCID: PMC1475757
- DOI: 10.1101/gad.378706
Combined ectopic expression of Pdx1 and Ptf1a/p48 results in the stable conversion of posterior endoderm into endocrine and exocrine pancreatic tissue
Abstract
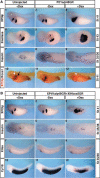
Patterning of the embryonic endoderm into distinct sets of precursor cells involves the precisely regulated activities of key transcription regulators. Ectopic, pan-endodermal activation of XPtf1a/p48 during pancreas precursor cell stages of Xenopus embryogenesis results in an expansion of the pancreatic territory, precisely within the borders of XlHbox8 expression. A combination of both activities is sufficient to expand the pancreatic precursor cell population also into more posterior portions of the endoderm. Both treatments result in the formation of a giant pancreas that persists up to late tadpole stages of development and carries both supernumerary endocrine and exocrine cells. A combination of XPtf1a/p48 and XlHbox8 is thus sufficient to convert nonpancreatic endodermal cells into pancreatic precursor cells.
Figures




References
-
- Afelik S., Chen Y., Pieler T. Pancreatic protein disulfide isomerase (XPDIp) is an early marker for the exocrine lineage of the developing pancreas in Xenopus laevis embryos. Gene Expr. Patterns. 2004;4:71–76. - PubMed
-
- Ahlgren U., Jonsson J., Edlund H. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development. 1996;122:1409–1416. - PubMed
-
- Ber I., Shternhall K., Perl S., Ohanuna Z., Goldberg I., Barshack I., Benvenisti-Zarum L., Meivar-Levy I., Ferber S. Functional, persistent, and extended liver to pancreas transdifferentiation. J. Biol. Chem. 2003;278:31950–31957. - PubMed
-
- Bort R., Zaret K. Paths to the pancreas. Nat. Genet. 2002;30:85–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
