Molecular dynamics study of MscL interactions with a curved lipid bilayer
- PMID: 16751236
- PMCID: PMC1544281
- DOI: 10.1529/biophysj.106.080721
Molecular dynamics study of MscL interactions with a curved lipid bilayer
Abstract
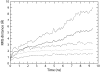
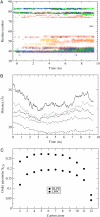
Mechanosensitivity is a ubiquitous sensory mechanism found in living organisms. The simplest known mechanotransducing mechanism is found in bacteria in the form of the mechanosensitive membrane channel of large conductance, MscL. This channel has been studied extensively using a variety of methods at a functional and structural level. The channel is gated by membrane tension in the lipid bilayer alone. It serves as a safety valve protecting bacterial cells against hypoosmotic shock. MscL of Escherichia coli embedded in bilayers composed of asymmetric amounts of single-tailed and double-tailed lipids has been shown to gate spontaneously, even in the absence of membrane tension. To gain insight into the effect of the lipid membrane composition and geometry on MscL structure, a fully solvated, all-atom model of MscL in a stress-free curved bilayer composed of double- and single-tailed lipids was studied using a 9.5-ns molecular dynamics simulation. The bilayer was modeled as a domed structure accommodating the asymmetric composition of the monolayers. During the course of the simulation a spontaneous restructuring of the periplasmic loops occurred, leading to interactions between one of the loops and phospholipid headgroups. Previous experimental studies of the role of the loops agree with the observation that opening starts with a restructuring of the periplasmic loop, suggesting an effect of the curved bilayer. Because of limited resources, only one simulation of the large system was performed. However, the results obtained suggest that through the geometry and composition of the bilayer the protein structure can be affected even on short timescales.
Figures

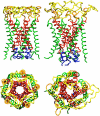

References
-
- Martinac, B. 2004. Mechanosensitive ion channels: molecules of mechanotransduction. J. Cell Sci. 117:2449–2460. - PubMed
-
- Hamill, O., and B. Martinac. 2001. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 81:685–740. - PubMed
-
- Häse, C., A. Le Dain, and B. Martinac. 1995. Purification and functional reconstitution of the recombinant large mechanosensitive ion channel (MscL) of Escherichia coli. J. Biol. Chem. 270:18329–18334. - PubMed
-
- Maurer, J., and D. Dougherty. 2003. Generation and evaluation of a large mutational library from the Escherichia coli mechanosensitive channel of large conductance, MscL: implications for channel gating and evolutionary design. J. Biol. Chem. 278:21076–21082. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

