Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity
- PMID: 16751269
- PMCID: PMC1482614
- DOI: 10.1073/pnas.0603161103
Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity
Abstract
Recent studies by our group and others demonstrated a required and conserved role of Stim in store-operated Ca(2+) influx and Ca(2+) release-activated Ca(2+) (CRAC) channel activity. By using an unbiased genome-wide RNA interference screen in Drosophila S2 cells, we now identify 75 hits that strongly inhibited Ca(2+) influx upon store emptying by thapsigargin. Among these hits are 11 predicted transmembrane proteins, including Stim, and one, olf186-F, that upon RNA interference-mediated knockdown exhibited a profound reduction of thapsigargin-evoked Ca(2+) entry and CRAC current, and upon overexpression a 3-fold augmentation of CRAC current. CRAC currents were further increased to 8-fold higher than control and developed more rapidly when olf186-F was cotransfected with Stim. olf186-F is a member of a highly conserved family of four-transmembrane spanning proteins with homologs from Caenorhabditis elegans to human. The endoplasmic reticulum (ER) Ca(2+) pump sarco-/ER calcium ATPase (SERCA) and the single transmembrane-soluble N-ethylmaleimide-sensitive (NSF) attachment receptor (SNARE) protein Syntaxin5 also were required for CRAC channel activity, consistent with a signaling pathway in which Stim senses Ca(2+) depletion within the ER, translocates to the plasma membrane, and interacts with olf186-F to trigger CRAC channel activity.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
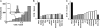

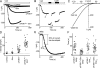


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

